|
 Implant Features
Implant Features
• Inserted via a
minimally-invasive posterior approach
• Radiolucent PEEK material with radiographic markers
• Potential for less patient morbidity; faster recovery
• Broad size range to match varying anatomy (11mm to 16mm diameter
available)
• Sphere restores disc space height and sagittal balance
• Fits within the natural concavity of the vertebral bodies
• May lessen adjacent-level degeneration
• Rests in the anatomic center of rotation
• Retains or restores motion
• Restores load transmission through vertebral body endplates
 Clinical
history on similar devices
Clinical
history on similar devices
Dr. Ulf Fernström of Uddevalla,
Sweden, was the first to publish the results of replacing a disc
with a mechanical prosthesis. Now known as the Fernström Ball, the
prosthesis consisted of a solid stainless steel sphere, implanted
through a posterior approach following a lumbar discectomy.
Between 1962 and 1964, Dr. Fernström replaced 191 lumbar discs in
125 cases. In 1966, he published the two year results from 105 of
the lumbar cases. In his report, Dr. Fernström compared the results
of disc replacement with his steel ball to a control group of 100
discectomies, both of which he followed for five to eight
years.1 He divided the cases into two groups: Group I consisted of
patients with a herniated disc, and Group II consisted of patients
with disc degeneration (i.e., positive discography and no root
compression).
At two-year follow-up, 12% of the patients who underwent disc
replacement and 60% of those who underwent discectomy in Group I
reported back pain. In Group II , 40% of the disc replacement
patients and 88% of the discectomy patients reported back pain. In
Group I, 14% of the disc replacement patients and 50% of the
discectomy patients reported sciatica. In Group II , 47% of the disc
replacement patients and 80% of the discectomy patients reported
sciatica.
Dr. Fernström also reported total disc collapse occurred in 0% of
the disc replacement patients and in 48% of the discectomy patients.
Dr. Fernström concluded that the results obtained as a result of
disc replacement were better than those achieved as a result of
discectomy, and that the results were similar to the results of
discectomy combined with fusion.
 Preoperative planning and patient positioning
Preoperative planning and patient positioning
The patient is placed on the
operating table in the prone position, making sure to allow enough
room for lateral fluoroscopy (Figure 1). A midline incision provides
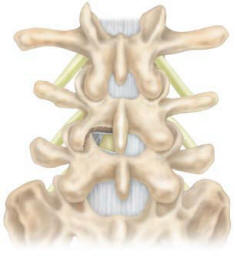
the approach and unilateral exposure of the interlaminar space and
facet joints at the affected level. A hemi-laminectomy is performed
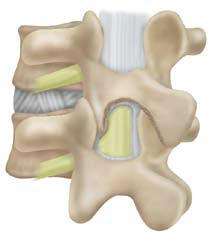
to expose both the dura and annulus lateral to the dura (Figure 2).
If needed, the spinous process can be undercut to gain access
(Figure 3).
Care should be taken to identify the lateral dura and traversing
nerve root at the affected level. The epidural veins are coagulated
over the annulus, and any other tethering of the traversing root is
dissected to allow for sufficient retraction of the dura and root.
 |
 |
 |
| Figure-1 |
Figure-2 |
Figure-3 |
 Distraction and disc removal
Distraction and disc removal
Distraction can be achieved with a
lamina or interspinous spreader. Using the table frame to place the
patient in a kyphotic position may provide an adequate opening of
the disc space (Figure 4).
While protecting the dura and nerve root, perform a discectomy by
incising the annulus lateral to the dura. Use the Pituitary Rongeur
to remove the excised annulus and nuclear material. Remove any
extruded fragments to decompress the neural elements and provide
entry to the disc space for distraction with minimal or no nerve
root retraction.
|
 |
|
Figure-4 |
 TRAILS
TRAILS
Trials are used to confirm the
appropriate final sphere diameter. The Trials are tapered on the
leading and trailing edge for easier insertion and removal from the
disc space. Align the flat ends of the Trial parallel to the
endplates. Lightly tap the trial into the disc space and turn the
handle 90° to assess the fit. The trial should fully engage the
endplates and restore the disc space to its normal height (Figure
6). Lateral fluoroscopic imaging is helpful to assess the fit of the
Trial.
To remove the Trial, turn the handle 90°, attach the Slap Hammer to
the Extension Handle and gently tap it to remove the Trial from the
disc space. Continue to use sequentially larger Trials until the
final sphere diameter is determined.
 FINAL
PREPARATION
FINAL
PREPARATION
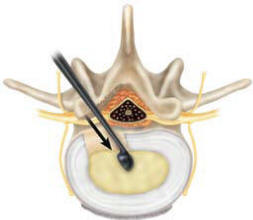
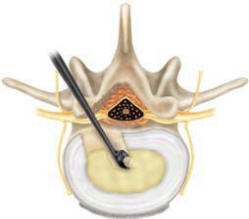
The Sphere curettes can be used to
remove any excess nucleus material. Using a curette equal to the
final Trial diameter, insert the curette into the disc space with
the smooth side oriented toward the surrounding neural structures.
Care should be taken to preserve the endplates where possible.
Position the curette at the desired location of the implant.
Generally, this is in the center of the disc space and slightly
posterior to midline (Figure 5a). Turn the Curette 360° to remove
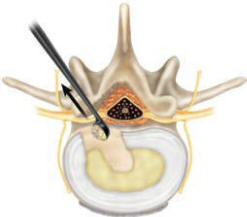
excess nucleus (Figure 5b). Remove the instrument by orienting the
smooth side of the Curette toward the neural structures and gently
removing it from the disc space (Figure 5c).
 |
 |
 |
| Figure-5a |
Figure-5b |
Figure-5c |
 Implantation
Implantation
Thread the appropriately sized
sphere onto the Inserter (Figure 6). Slide the Inserter’s outer
sleeve forward so the pin engages the small anti-rotation dimple on
the sphere and tighten the wingnut to lock into place. Gently impact
the sphere into the disc space by orienting the sphere toward
midline of the disc space (Figure 6). Once the sphere is fully
seated, turn the Inserter’s handle counter-clockwise to disengage
the implant. The Angled graft impacter can be used to achieve the
desired position of the implant. In the event that the implant needs
to be removed, thread the inserter into the implant and remove.
 Indications
Indications
The SATELLITE System PEEK Nucleus
Replacement is indicated for use in skeletally mature patients (at
least 17 years old) undergoing surgery of the lumbar spine for
symptomatic degenerative disc disease.
 Contraindications
Contraindications
The SATELLITE System PEEK Nucleus
Replacement should not be implanted in patients who have an active
infection, or who are pregnant. Nor should the device be used in
patients who do not meet the criteria listed above.
 Postoperative course
Postoperative course
Gradually increase your activity.
May be required to wear a back brace after surgery and to avoid
repetitive bending, lifting, twisting, and athletic activities while
recovering. Cautioned to avoid vibrations, like experience when
driving a car, for a period of time after surgery.
Contact your doctor immediately if:
• There is a fever
• The incision starts leaking fluids
• There is trouble swallowing or breathing
• There is trouble urinating
• New or increased back or leg pain or numbness |