|
The
management of a
patient suffering
from an injured
peripheral nerve
requires an
understanding of the
mechanics of injury,
the pathological
response, and
subsequent
regenerative
capacity. Decisions
concerning whether
to operate, when to
operate, and what to
do once the lesion
is exposed must be
based upon not only
a firm understanding
of the pathology of
the repair but also
some acceptance of
the limitations for
neural regeneration
in terms of
practical functional
recovery. Clinical
examination,
electrodiagnostic
studies, and
radiologic studies
are helpful in
making such
decisions. Patient
selection for
operation as well as
timing, type(s) of
operation, and the
value of operation
persist as
controversial
issues.
 Guidelines for
Injury Evaluation
Guidelines for
Injury Evaluation
The
major determinants
for deciding whether
or not to operate on
injured nerves are
(1) the mechanism of
injury, (2) the
severity of the
neurological loss,
and (3) the presence
of severe pain.
Sharp or blunt
lacerations
involving soft
tissues and nerve(s)
with severe distal
loss will require
operation. Blunt
injuries associated
with stretch,
fracture, contusion,
compression, and
even gunshot wound
(GSW) are more
likely to preserve
some physical
continuity of the
involved nerve and
may or may not
improve without
operation.
If
loss is complete
distal to the
injury, complete
improvement with
time is less likely
but can, on
occasion, still
occur. When loss is
incomplete and
continuity of the
nerve is likely
because of the
mechanism of injury,
function will
usually improve with
time. There are, of
course, exceptions:
(1) when the
partially injured
nerve, although in
continuity, is
compressed by a
pseudoaneurysm or
expanding clot when
the site of nerve
injury is close to
an area of potential
entrapment- e.g.
ulnar nerve at the
olecranon notch,
median nerve at the
wrist, posterior
interosseous nerve
in the region of the
supinator, or
peroneal nerve at
the head of the
fibula.
Although
regeneration
following proximal
nerve lesions is
faster than that
which follows distal
injury, axons must
traverse great
distances to reach
distal target sites.
Thus, in most cases,
gaining good results
is more difficult
following proximal
lesions than distal
ones and delays in
their repair should
be avoided.
 Axonal Regeneration
Considerations
Axonal Regeneration
Considerations
The
injured peripheral
nerve has
characteristic
neuronal and axonal
responses. The
severity of injury
will partly
determine the degree
of axonal
regeneration.
Although the rate of
axonal growth and
maturation of motor
function is slow,
the rate of
regeneration is
predictable.
Regeneration
proceeds at the rate
of 1 mm per day or 1
inch per month. This
helps the physician
establish
approximate
deadlines in
relationship to time
of injury or
previous repair in
expecting clinical
signs of
reinnervation.
If
the first target
muscle begins to
show function at the
expected time and
power improves over
the next 1-2 months,
the decision against
surgery is clear. If
the expected time
schedule is not met,
or the subsequent
early quantitative
extent of motor
activity in the
first target muscle
does not match the
expectancy after
repair, operative
intervention is
indicated.
Unfortunately, too
much time is
required for many
nerve lesions to
reach even early
regenerative
milestones. Under
these circumstances,
if repair is delayed
until after these
deadlines are met,
results are not as
good as with earlier
repair.
The
time required for
regeneration
involves the
following
considerations:
1.
There is a delay
before regenerating
axons reach the
nerve distal to
either injury or
suture repair. The
segment of
retrograde
degeneration
proximal to the
injury must first be
overcome, and then
there is usually a
delay of 1-2 weeks
before axons
penetrate the injury
or repair site and
reach the distal
stump. This period
of delay may be 2-4
weeks.
2.
Once the fibers have
reached the distal
stump, the rate of
axonal growth
decreases as the
distance of the
injury from the
neuron increases.
3. A
terminal delay of
weeks to several
months takes place
between the time
when axons reach
their distal targets
and when sufficient
maturation of the
axons and their
receptors occurs to
allow maximal
function. Thus, it
is not enough for
axons to reach their
distal targets; they
must do so in
sufficient number
and with enough
caliber and
myelination to
produce acceptable
function.
Evidence of
regeneration, as
gauged by return of
nerve function, can
help guide the
initial management
of such lesions.
Positive evidence
for some significant
nerve function,
either initially or
within 6 weeks
postinjury, implies
a favorable result.
When a significant
proportion of axons
have escaped initial
dysfunction or have
suffered only a
minor degree of
nerve fiber injury,
regeneration occurs,
exceeding the best
that nerve repair
could yield.
The
more frequent
clinical situation
is that of total
nerve dysfunction in
which the lesion has
not been operatively
inspected, or in
which exposure at
surgery has revealed
a neuroma in
continuity. If a
nerve repair has
been performed
elsewhere under
uncertain
circumstances, a
similar management
dilemma arises. In
these cases, delayed
surgical exploration
with intraoperative
nerve action
potential recordings
is invaluable in
making the final
decision regarding
resection and repair
of the damaged
nerve.
 Clinical Evaluation
Clinical Evaluation
 Motor Examination
Motor Examination
A
point to stress
regarding clinical
motor examination
for specific nerve
injuries is that the
single most
important step in
management of any
nerve injury is a
detailed examination
of the limb, with
careful grading of
all motor and
sensory function.
The examination must
then determine
whether loss is
complete or
incomplete distal to
the injury site.
Only in this fashion
can one can tell on
subsequent
examinations whether
or not function has
changed.
Motor
examination is
sufficient by itself
as proof of
regeneration when
recovery is obvious.
Clinically observed
voluntary motor
function can also be
confirmed by motor
response to nerve
stimulation. Nerve
stimulation is
especially helpful
in early recognition
of adequate peroneal
recovery and
avoidance of a
needless operation.
Patients with injury
to the peroneal
nerve are unable to
initiate voluntary
action in the
peroneus and
anterior tibial
muscles (eversion
and dorsiflexion of
the foot). This may
continue for several
weeks after
electrophysiologic
recovery has been
demonstrated by
strong muscle
contraction on
peroneal nerve
stimulation: (1)
just behind the head
of the fibula or,
(2) just inside the
lateral hamstring,
where the nerve
trunk is readily
palpated.
Importantly, one
must be certain that
the muscle observed
to contract is in
the distribution of
the nerve presumed
to be stimulated.
 Tinel's Sign
Tinel's Sign
If
paresthesias are
obtained by
percussion of nerve
distal to the
injury, there is a
suggestion that some
sensory axons are
continuous from the
point percussed
through the lesion
to the central
nervous system. If
the response moves
further distally
with time, and
especially if this
is associated with
diminished
paresthesias in
response to tapping
over the injury
site, evidence of
continued sensory
fiber regeneration
down the distal
stump is present
(positive Tinel's
sign). A positive
Tinel's sign,
however, implies
only fine fiber
regeneration and
tells the examiner
nothing about the
quantity and
eventual quality of
the new fibers.
On
the other hand,
total neural
interruption is
strongly suggested
by an absence of
distal sensory
response (negative
Tinel's sign) after
adequate time has
elapsed for fine
fiber regeneration
to occur (4-6
weeks). A negative
Tinel's sign is more
valuable in clinical
evaluation than a
positive Tinel's
sign.
 Sweating
Sweating
Return of sweating
in an autonomous
zone signifies
sympathetic nerve
fiber regeneration.
This return may
antedate sensory or
motor return by
weeks or months,
since autonomic
fibers regenerate
rapidly. Return of
sweating does not
necessarily mean
that sensory or
motor function will
follow.
 Sensory Recovery
Sensory Recovery
True
sensory recovery is
a useful sign,
especially when it
occurs in autonomous
zones where overlap
from adjacent nerves
is minimal.
Autonomous zones for
the median nerve
include the volar
and dorsal surfaces
of the forefinger
and volar surface of
the thumb. The
radial nerve does
not have a reliable
autonomous zone. If
there is any sensory
loss in its
distribution, it
will usually involve
the region of the
anatomic snuff box.
The autonomous zone
for the ulnar nerve
includes the palmar
surface of the
distal 1.5 phalanges
of the little
finger. Autonomous
zones for the tibial
nerve include the
heel and a portion
of the sole of the
foot, while for the
peroneal nerve it
includes mid-dorsum
of the foot.
Unfortunately,
sensory recovery,
even in an
autonomous zone,
does not ensure
subsequent motor
recovery.
 Electrophysiologic
Studies
Electrophysiologic
Studies
 Electromyography
Electromyography
A
thorough baseline
electromyographic
(EMG) study (Figure
1A) 2-3 weeks
following the injury
will document the
extent of
denervation and will
confirm the pattern
or distribution of
the injury. EMG
studies should be
done serially to
search for signs of
reinnervation or
persistence of
denervation. With
regeneration,
insertional activity
will begin to return
and the fibrillation
and denervation
potentials will
decrease in number
and sometimes be
replaced with
occasional nascent
motor action
potentials. Such
changes indicate
that some
regenerating fibers
have reached muscle
and that some
axon-to-motor end
plate connections
have been
reconstructed.
These
signs tell nothing,
however, of the
eventual extent or
quality of
regeneration.
Nonetheless, when
decreased numbers of
fibrillations as
well as nascent
potentials are found
in muscles in the
distribution of an
injured nerve, a
short interval of
further conservative
management is
suggested. The EMG
is important because
it can give evidence
of regeneration
weeks or months
before voluntary
motor function is
detectable. It can
also detect retained
motor units to
indicate a partial
lesion early after
injury.
The
EMG is particularly
helpful in defining
the level of injury
in a brachial plexus
lesion and thus in
selecting patients
for operation as
well as the type of
operation to be
used. Paraspinal
muscle denervation
suggests a proximal
lesion(s) to one or
more roots and thus
is a negative
finding. Proximal
damage to the lower
three roots can
result in extensive
paraspinal
denervation while
the C5 and even the
C6 roots may be more
laterally injured
and are, thus,
repairable. The
electromyographer
has difficulty
sampling distinct
spinal levels within
the paraspinal
muscle because there
is so much overlap.
An
operation is usually
indicated in
brachial plexus
lesions if complete
loss in the
distribution of one
or more upper roots
(C5,C6,C7) and their
distal outflows does
not begin to reverse
clinically or
electrically in the
early months
postinjury.
The
presence of EMG
changes suggesting
reinnervation does
not guarantee
recovery of
function, and the
test must be weighed
in conjunction with
clinical findings
and other electrical
data. Because the
EMG can continue to
show quite severe
denervational
changes even though
the muscle contracts
voluntarily, the EMG
should never be
substituted for a
careful clinical
examination. Rather,
it should supplement
the clinical
examination. EMG is
especially valuable
in identifying
anomalous
innervation, such as
occurs frequently in
the forearm and
hand.
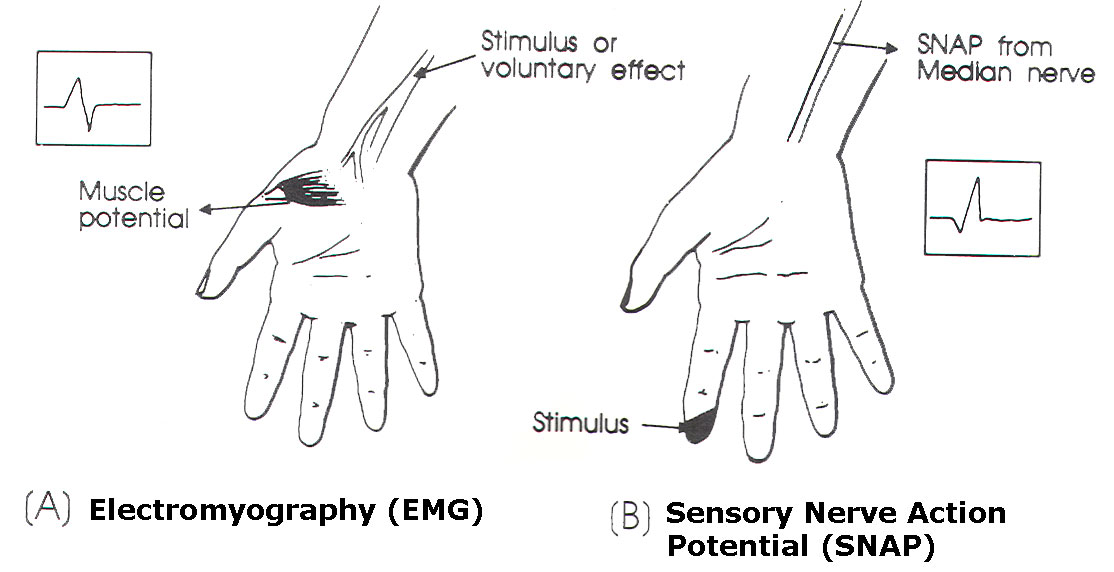
Fig-1 A (EMG) and B
(SNAP).
 Sensory Nerve Action
Potential (SNAP)
Sensory Nerve Action
Potential (SNAP)
SNAP
studies (Figure 1B)
can be helpful in
evaluating the level
of brachial plexus
stretch injuries.
Lesions at a root
level that are
restricted to the
preganglionic region
and do not extend
into the
postganglionic
region produce
complete distal
sensory loss and
preservation of
distal sensory
conduction. The
latter is preserved
because sensory
fibers damaged
distal to the dorsal
root ganglion do not
degenerate.
This
retention of sensory
conduction from an
anesthetic area can
be tested by
stimulating fingers
in the c6 (thumb and
index finger),
C67-8 (long
finger), and C8- T1
(little and ring
fingers)
distributions and
recording from the
median, radial, and
ulnar nerves
proximally. The
presence of a
compound sensory
nerve action
potential
substantiates a
preganglionic injury
in the distribution
of one or more
roots.
Since
even distal sensory
distributions of
roots overlap with
one or more other
roots, it is
difficult to be
certain by these
studies that one
root, C6 for
example, has a
preganglionic
injury.
Stimulation of an
anesthetic
forefinger (or even
thumb) can produce a
SNAP in the median
nerve distribution
if either C6 or C7,
or c6 and C7 roots,
are damaged at a
preganglionic level.
This makes it
difficult to
determine by SNAP
studies whether or
not the C6 root has
incurred a
preganglionic
injury. The
situation is even
less favorable for
the C5 root since
there are no
specific noninvasive
stimulation or
recording sites for
this outflow:
Detailed evaluation
of upper roots by
SNAP recordings is
not possible at this
level.
 Somatosensory-Evoked
Potential (SSEP)
Somatosensory-Evoked
Potential (SSEP)
SSEP
study (Figure 1C)
has been used in
evaluating the level
of injury- i.e.
preganglionic versus
postganglionic-in
brachial plexus
lesions.
Somatosensory
studies have limited
value in the early
months following
injury.
Somatosensory
studies can,
however, be used at
the time of surgery
for stretch/
contusion brachial
plexus injuries. If
the injury is
postganglionic,
stimulation of the
root proximal to the
level of the injury
should evoke a
somatosensory
potential over the
cervical spine (SSP)
and an evoked
cortical response
over the
contralateral
cranium (ECR). If
the injury is
preganglionic or
pre- and
postganglionic,
stimulation of the
root, even within or
close to the
intervertebral
foramen, will evoke
no such responses.
Repair of at least
that element is
unlikely to be
successful.
Unfortunately,
production of an SSP
or ECR probably
requires only a few
hundred or so intact
fibers between site
stimulated and site
recorded, so a
positive response
only ensures minimal
continuity of spinal
nerve or root. A
negative ECR is of
more importance than
a positive ECR.
 Intraoperative Nerve
Action Potential
(NAP)
Intraoperative Nerve
Action Potential
(NAP)
NAP
study (Figure 1D)
involves operative
exposure of the
nerve trunk on
either side of the
lesion. Since one
ideally seeks to
decide whether to
repair a nerve by 8
weeks after injury,
NAP becomes an
important definitive
test when gross
appearance of a
neuroma in
continuity is
equivocal and the
first target muscle
is more than 3
inches downstream.
|
|
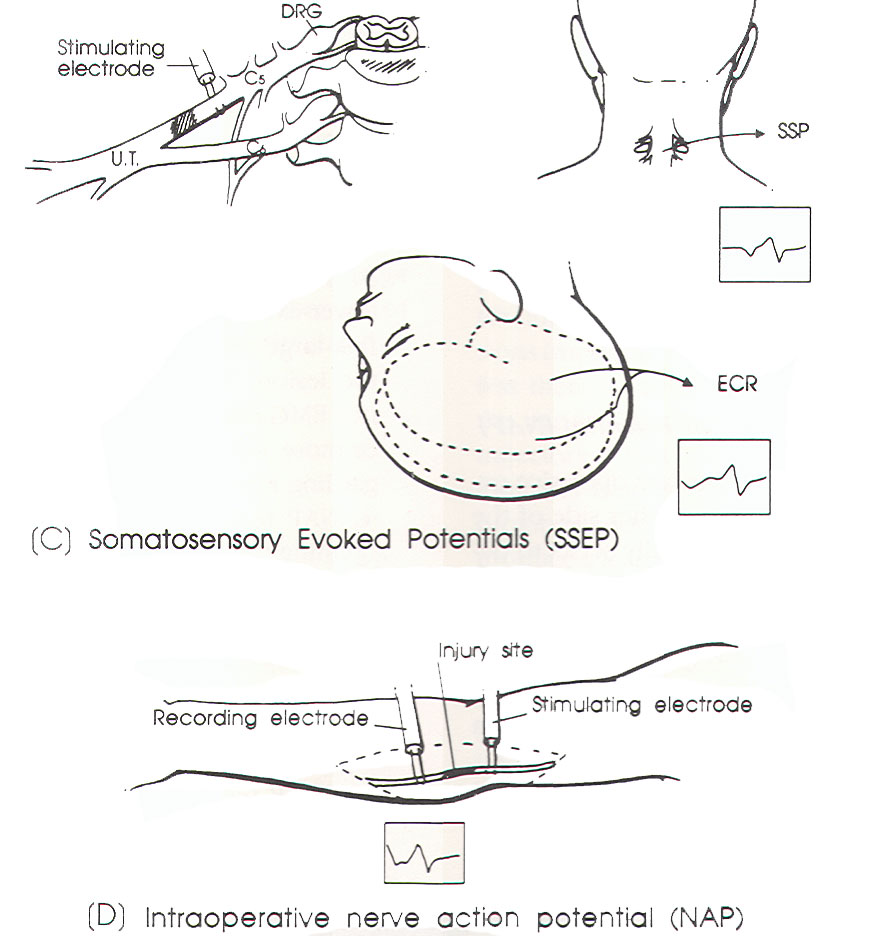 |
|
Fig-1 (C
and D) |
The
important
considerations with
NAP recordings are:
1.
The gross appearance
of a neuroma in
continuity does not
necessarily
correlate with the
internal
architecture.
2. If
axons have been
given an opportunity
to traverse the
lesion, their
presence may be
recorded by the NAP
long before those
axons have had an
opportunity to reach
their end target.
3.
This technique is
particularly useful
in lower-extremity
nerve lesions in
which the first
target muscle may
lie 6-8 inches below
the lesion. Thus,
neither nerve
stimulation nor EMG
can settle the issue
for 6-8 months or
more, but it is
important that
decisions regarding
resection be made
before that time.
4.
NAP recordings are
also very helpful in
defining the extent
of brachial plexus
lesions and provide
a useful index of
how much of the
proximal stump of
the lesion in
continuity to
resect. Most
brachial plexus
injuries selected
for operation will
have one or more
elements in
continuity. but with
a variable amount of
intraneural damage.
Intraoperative NAP
recording helps sort
out the need for
resection.
At
surgery, the
critical observation
is whether or not
there is a
recordable response,
and not its form or
even its velocity.
Regenerative NAP
responses are small
and usually slow,
while those due to
partial sparing may
be small but are
usually faster or
have conduction in a
normal range. Where
there has been
preganglionic
without
postganglionic
injury, more distal
recording will show
a rapid conducting,
large NAP, which is
just as diagnostic
as absence of an SSP
or ECR when the root
is stimulated at
that level.
 Radiologic Studies
Radiologic Studies
 Cervical Spine and
Other X-rays
Cervical Spine and
Other X-rays
Cervical spine
fractures are
frequently
associated with
severe proximal,
irreparable stretch
injuries, at least
at the root levels
associated with
those vertebrae.
Fractures of other
bones such as the
humerus, clavicle,
scapula, and/or
ribs, when observed,
give rough estimates
of the forces
brought to bear on
the shoulder, arm,
and neck, but do not
necessarily help
localize the level
or document the
extent of the
injury. Damage to
the plexus is
usually more
proximal than the
fracture site would
indicate, frequently
at the root level.
Midhumeral fractures
are especially
associated with
radial nerve
injuries. Comminuted
fractures of the
radius and ulna at
midforearm level can
also be associated
with combined median
and ulnar nerve
injuries, and on
occasion with
posterior
interosseous nerve
palsy. The peroneal
component of the
sciatic nerve is
often, but not
always, selectively
involved in hip
dislocation or
fracture. Lower
femur fractures as
well as tibial and
fibular fractures
may involve the
peroneal and/or
tibial nerves. Once
again, the nerve
injury may be more
proximal than the
fracture(s) site(s)
may suggest. A
midshaft femur
fracture may be
associated with a
more proximal
sciatic stretch
injury at the
buttock level.
Chest
radiographs may
reveal elevation of
a nonfunctioning
diaphragm, which
denotes phrenic
nerve paralysis.
This is a relatively
poor prognostic sign
for repairability of
the C5 nerve root
following closed
injuries, because it
usually implies
proximal damage at
that level of the
neck.
 Myelography
Myelography
Myelography may be
an important part of
the work-up in a
patient with severe
brachial plexus
stretch injury. It
is usually not
indicated for
infraclavicular or
axillary level
plexus lesions (most
gunshot wounds to
the plexus), unless
there is radiologic
evidence of damage
to the cervical
spine or a medial
supraclavicular
trajectory. A
meningocele at a
given level
indicates that
enough force was
applied at a
proximal root level
to tear the
arachnoid and
produce a leakage of
contrast agent. It
does not necessarily
mean that the root
is avulsed out of
the spinal cord.
More commonly, the
presence of a
meningocele implies
that, although the
root may still be in
gross continuity, it
has significant
internal damage at a
very proximal level.
A
number of patients
have had successful
repair of roots at
levels where
meningoceles were
absent (usually
upper root levels),
despite meningoceles
on other roots
(usually lower
levels).
Nonetheless, if a
meningocele is
present, it is most
likely that the root
has proximal and
thus irreparable
damage. This finding
also makes it more
likely that damage
at other levels
without meningoceles
is very proximal.
Myelography may
delineate the
rootlets in the
subarachnoid space,
and comparison of
the affected and
unaffected sides may
delineate sites of
root disruption.
Myelography is still
a useful adjunct in
the decision-making
process concerning
plexus injuries.
 Computed Tomography
(CT) and Magnetic
Resonance Imaging
(MRI)
Computed Tomography
(CT) and Magnetic
Resonance Imaging
(MRI)
Computed tomography
(CT) scanning with
intrathecal contrast
is of interest in
stretch injuries,
although an
abnormality may
still be missed,
because slices could
be not thin enough
to cover all of the
root regions at each
level. As a result,
myelography still
remains the
preferred radiologic
study.
Magnetic resonance
imaging (MRI) may
help visualize the
nerve root. Such
studies can
supplement the
myelogram and
replace it.
Cerebrospinal fluid
(CSF) within
meningoceles can be
seen on MRI, but
usually with less
clarity than it is
visualized by
conventional
myelography.
 Guidelines for
Timing of Repair
Guidelines for
Timing of Repair
 General
Considerations
General
Considerations
In
deciding when to
repair, the surgeon
must define: (1)
when the time for
useful recovery by
spontaneous
regeneration has
passed, and (2) the
elapsed time when a
nerve repair has
little to offer.
When the duration of
total muscle
denervation exceeds
24 months ("24-month
rule"), most muscles
are subject to
relatively severe
time limitations for
the return of useful
function. This is
less likely to be so
for large bulky
muscles, such as
biceps and
gastrocnemius-soleus,
than for smaller
muscles, such as
those of the forearm
and hand. An
exception to this
guideline are the
facial muscles
which, although
relatively small,
may benefit from
late reinnervation
by facial nerve
repair or
neurotization
procedures.
Other
exceptions to the
"24-month rule" may
occur in a few
lesions that have
maintained some
nerve fiber
continuity. If some
fibers traverse the
lesion, even though
their number is
insufficient to
produce useful
function distally,
they may promote
distal stump
architecture
preservation. Very
late repair after
resection of the
lesion in continuity
can occasionally
produce function.
Distance from the
site of nerve injury
to the desired
muscle influences
the timing of
surgery. When the
site of injury is a
long distance from
an important muscle,
it is essential to
perform the repair
within a few months
postinjury. This is
especially so with
sciatic nerve and
brachial plexus
injuries.
Relatively early
repair of other
nerve injuries also
is beneficial. For
example, when the
radial nerve is
injured in
association with a
closed midhumeral
fracture, the
probability for good
spontaneous recovery
is high. Exploration
should be undertaken
if there is no
recovery by 4
months. By this
time, a midhumeral
axonotemetic injury
of the radial nerve,
should have
regenerated to the
next muscle
downstream, the
brachioradialis. If
the radial nerve is
seriously damaged
between the fracture
fragments, repairing
it much later would
begin to yield less
satisfactory results
for return of motor
function.
In
contrast, there
exist cases when the
distance between the
nerve injury and the
muscle to be
reinnervated is such
that repair, early
or late, will not
accomplish a useful
degree of motor
recovery. For
example, repair of
the ulnar nerve near
the axilla or
peroneal nerve above
the midthigh may
accomplish little in
the way of function
to important distal
muscles. Other high
repairs may be
indicated, however,
either because there
are useful proximal
muscles (such as the
triceps and proximal
forearm extensors in
case of radial
nerve) or because
sensory recovery is
valuable (such as
that in the
distribution of the
median nerve).
Time
limitation is less
severe in sensory
recovery than in
motor recovery. This
is an important
consideration in
favor of median
nerve repair as high
as the axillary
level, even though
it may only
contribute minimal
motor function at
the hand level. A
high median nerve
repair is especially
important if a
mechanically useful
hand can be provided
by substituting or
transferring some of
the ulnar and radial
motor function that
does exist.
Similarly, repair of
the tibial component
of the sciatic nerve
at a level as high
as the sciatic notch
may be indicated.
Protection to
weightbearing
plantar areas can be
given by even a
low-grade recovery
of sensory function.
Thus, restoration of
protective sensation
to the sole of the
foot is important
enough to warrant a
proximal repair.
Some degree of
useful plantar
flexion will usually
come about as well.
Finally, motor
recovery from
spontaneous
regeneration
(without nerve
repair) also has
limitations in time.
High nerve lesions
will usually result
in lack of useful
distal motor
function if the
muscle is over 24
inches distal to the
lesion and is
totally denervated
by the injury.
Relatively early
evidence for some
recovery of motor
function, even if
only detectable by
EMG, will greatly
improve the
prognosis. These
findings must be
evident by 2-3
months postinjury.
Therefore, in some
high lesions,
measures to
compensate for lost
motor function can
be taken quite
early. Tendon
transfers can be
performed without
awaiting the effect
of possible late
regeneration from a
high or proximal
ulnar nerve lesion.
 Early (Primary)
versus Delayed
(Secondary) Repair
Early (Primary)
versus Delayed
(Secondary) Repair
Early
operative
intervention is
infrequently needed
in most peripheral
nerve stretch
injuries. There are
notable exceptions.
An enlarging
hematoma or
aneurysmal sac will
convert a partial
incontinuity nerve
injury into a
complete and, with
time, irreversible
lesion unless the
mass is removed as
early as possible. A
severely contused
forearm or a distal
humeral fracture
associated with
brachial artery
injury predisposing
to Volkmann's
ischemic contracture
are other exceptions
regarding delay of
an operation. In
this instance, early
fasciotomy,
treatment of the
vascular injury, and
in many cases
neurolysis of one or
more nerves are
necessary.
A
similar syndrome can
involve the anterior
compartment of the
lower leg, requiring
urgent intervention
if irreversible
neural as well as
muscular changes are
to be avoided. A
severe noncausalgic
pain syndrome
secondary to a
missile embedded in
nerve also can
benefit from an
early operation and
removal of the
missile fragment.
Injury to nerve in
areas of potential
entrapment may also
require early
release of the nerve
and section of the
connective tissue
structures likely to
cause entrapment.
This is done to
avoid potentially
irreversible changes
in the nerve.
Early
(primary) repair is
a valid option for
the repair of
simple, clean
lacerating injuries
such as those caused
by glass and knives.
In civilian
injuries, primary
repair is best for
sharply transected
supraclavicular and
axillary level
brachial plexus and
sciatic nerve
injuries; immediate
exploration provides
the best opportunity
for both accurate
identification and
end-to-end repair
without need for
grafts.
This
is especially so
with sharp plexus
injuries in which
there is vascular
damage that must be
repaired at once. If
such a wound site is
explored some weeks
later. one is
usually confronted
by heavy scar that
makes dissection and
identification of
involved neural
elements difficult.
At the time of
exploration. one
must make sure that
the transection is
sharp and clean
before a primary
repair is done, when
confronted with a
transected nerve.
the following
factors favor
primary repair:
1.
Nerve stumps are
easy to locate and
their relationships
to other injured
structures usually
are preserved.
2.
Nerve stumps are
minimally retracted.
3. A
single operative
procedure is
definitive and may
be the only
operation necessary
to repair soft
tissue as well as
nerve injury.
Primary repair
should only be
undertaken by
surgeons who have
total mastery of the
anatomy of the
injured region and
who have been
trained in macro-
and microperipheral
nerve surgical
techniques.
Not
all transecting
injuries lend
themselves to
primary repair. If
the ends are ragged
or contused. a
delayed repair is
preferable. In this
case. the surgeon
cannot know how much
of either stump to
resect in order to
get back to healthy
neural tissue. Even
with injuries caused
by sharp objects, a
contused rather than
transected nerve can
result, and delayed
repair then becomes
mandatory. If
clinical or
substantial EMG
recovery does not
occur in the first
2-3 months,
reoperation to
evaluate the lesion
in continuity and to
make a decision for
or against resection
and repair is
indicated.
In
summary, the
arguments favoring
delayed or secondary
repair include:
1.
Damage to the
proximal and distal
stumps has had time
to be defined by
visible intraneural
scarring on
cross-section. The
surgeon can then be
certain that
resection back to
normal neural tissue
is accomplished.
2.
Associated injuries
have had a chance to
heal, infection has
been minimized, and
the
patient has learned
to use the extremity
before being
subjected to
operation and
sometimes to
immobilization and
its attendant
discomfort.
3.
The epineurium has
thickened so as to
allow easier
placement of
epineural sutures.
4.
Operation is
elective and can be
performed
accurately.
5.
The distal stump is
cleared of
degenerating
axoplasm and myelin.
6.
Approximately 15% to
20% of lacerations
have not transected
the nerve or nerves
in a limb with
associated complete
loss of function in
the distribution of
one or more nerves.
It is impossible to
decide immediately
postinjury whether
or not to resect
such a lesion in
continuity.
When
the nerve is not
known to be
transected (closed
injury) but is
without function,
especially following
high-velocity
missile wounding as
shown in Figure 1,
delayed (secondary)
repair is indicated.
The majority of
closed injuries to
the nerve are due to
stretch/ contusion.
The nerve is not
divided and there is
a variable degree of
intraneural damage.
This may be a
mixture of
axonotmesis,
neurotmesis and
neuropraxia, or may
be due to complete
neurotmesis. Thus, a
delay of several
months is necessary,
since this will
permit (1) any
element of
neuropraxia to
resolve, (2)
associated injuries
to heal, and (3)
most importantly,
physiologic
evaluation of the
lesion at the
operating table. If
adequate
regeneration is
occurring,
spontaneous activity
can be detected by
means of
intraoperative NAP
recording techniques
by 8-10 weeks
postinjury.
If a
NAP is present, the
nerve will fare well
with simple
neurolysis. Most
often, external
neurolysis is
performed. This
consists of freeing
the nerve from
surrounding tissue,
including scar, and
exposing the entire
circumference of the
nerve. Internal
neurolysis,
involving resection
of scar tissue away
from the nerve
fascicles, is
usually reserved for
certain partial
nerve lesions
requiring split
repair and
management of
refractory neuritic
pain. Figure 5 shows
a high peroneal
division split
repair.
Neurolysis may or
may not assist in
continued
regeneration and
hasten recovery.
Some authors believe
that adhesions and
scar tissue can
obstruct or delay
the growth of
regenerating axons
and even block
conduction in nerve
fibers. Other
investigators say
that recovery under
these circumstances
would have occurred
even if neurolysis
had not been done.
Neurolysis may also
relieve or
ameliorate
noncausalgic
neuralgic pain by
removing adhesions
or constricting scar
that fix and at
times deform the
nerve. Neurolysis or
nerve repair is less
likely to ameliorate
pain in nonfocal
injuries, such as
stretch-contusion,
particularly of the
plexus. A daily
regimen of
carbamazepine and
amitriptyline may
help relieve pain.
Vigorous physical
therapy is essential
in pain management.
Early mobilization
of the involved limb
should be stressed
to the patient and
family. Reassurance
of the temporary
nature of the pain,
at least in patients
with acute nerve
injury, is also
helpful.
Occasionally,
patients may benefit
from transcutaneous
peripheral nerve
stimulation devices.
If a
NAP is not present
and 8 weeks have
elapsed, recovery
will not occur
unless resection
back to healthy
neural tissue and
repair are
performed. An
end-to-end repair is
preferred. Use of
autologous grafts,
usually using the
sural nerve, is the
method of choice for
bridging a gap that
cannot be closed
without tension by
an end-to-end union.
The success of nerve
grafting declines as
the length of the
graft increases,
usually because the
lengthier injuries
requiring longer
grafts are more
severe.
Grafting very long
defects under
unfavorable
conditions is not
worthwhile in some
nerves, because the
chances of obtaining
any useful function
are remote. In such
cases, alternative
methods such as
neurotization
procedures should be
considered. These
include use of the
cervical plexus,
accessory nerve, or
intercostal nerves
as proximal outflow
to attach to sural
grafts. Such
procedures have
sometimes provided
either shoulder
abduction or biceps/
brachialis
contraction.
Neurotization has
difficulty
substituting for
loss of more than
one function,
although a recent
report in a small
number of patients
suggests that this
may still be a
possibility.
After
it is clear that
recovery is
unlikely, an injured
nerve should be
repaired with the
least possible delay
This minimizes
distal nerve trunk
and fascicular
atrophy which will
lead to poor
results. According
to Sunderland, such
atrophy is evident
by the end of the
first month
postinjury, reaches
a peak somewhere
between the third
and fourth months,
and then levels out.
As a general
guideline, focal
lesions in
continuity (those
associated with
fracture, soft
tissue contusion,
and some gunshot
wounds) can be
accurately evaluated
intraoperatively at
2-3 months
postinjury.
Stretch or severe
contusion injuries
(those associated
with vehicular and
skiing accidents,
falls, crush
injuries, and
shotgun pellets)
produce lengthier
lesions and need to
be followed longer
to assess full
regenerative
capacity These
lesions can usually
be accurately
evaluated
intraoperatively by
electrical
recordings at 3-5
months
postoperatively.
Delay in referral,
healing of
associated injuries,
and management of
infection may alter
the timing of
operation. Despite
these guidelines for
lesions in
continuity, when one
is in doubt about
the direction of
recovery, it is
better to assess the
nerve directly.
Excessive delay in
nerve repair leads
to poor results.
Table 1 summarizes
these guidelines for
lesions in
continuity.
|
TABLE 1
Management
of the
Neuroma
in
Continuity |
|
Incomplete
loss
with
significant
distal
sparing |
|
1. |
Most
cases
will
improve
with
conservative
treatment.
They are
followed
by
serial
clinical
and EMG
examinations. |
|
|
Physical
therapy
is
important. |
|
2. |
Operation
may
still be
required: |
|
|
a. |
Partial
lesions
associated
with
expanding
masses
due to
hematoma,
aneurysm,
or A-V
fistula
usually
require
urgent
operation. |
|
|
b. |
Partial
lesions
close to
or in
areas of
potential
entrapment
may
require
relatively
early
operation. |
|
|
c. |
Lesions
where
distal
loss,
although
partial,
is
significant
may
require
later
operation. |
|
|
d. |
Neural
pain not
amenable
to
medications
and
physical
therapy
may
require
later
operation. |
|
Complete
or near
complete
loss
with
little
or no
distal
sparing |
|
1. |
Relatively
focal
lesions
in
continuity
due to
fracture
or
gunshot
wound. |
|
|
a. |
Follow
by
clinical
and EMG
examinations
for 2-3
months. |
|
|
b. |
If no
significant
clinical
or
electrical
improvement,
explore. |
|
|
c. |
Intraoperative
stimulation
and NAP
studies
used to
decide
for or
against
resection. |
|
2. |
Relatively
lengthy
lesions
in
continuity
due to
stretch/contusion
or
shotgun |
|
|
a. |
Follow
by
clinical
and EMG
examinations
for 4-5
months. |
|
|
b. |
If no
significant
clinical
or
electrical
improvement,
explore. |
|
|
c. |
If no
response
to
stimulation
and no
NAP
across
lesion,
resection
and
repair
by
suture
or graft
are
necessary. |
|
|
d. |
Intraoperative
evoked
cortical
or
somatosensory
studies
may be
necessary
to
evaluate
repairability
of
proximal
spinal
nerves.
(See
Table
2). |
 Selection of
Patients for Surgery
Selection of
Patients for Surgery
Despite the
guidelines outlined
above, controversy
concerning the value
and timing of
surgery as well as
patient selection
continues to exist.
This is especially
evident in the
management of
brachial plexus
stretch/contusion
injuries, Some
authors consider few
or none of these
injuries suitable
for surgery, while
others believe that
all stretch injuries
should be explored.
This brings one to
the question: How
does one select
patients with
brachial plexus
stretch lesions for
surgery? Table 2
summarizes some of
the criteria
currently used for
selection.
Very
proximal injury
involving the roots
close to the spinal
cord and/or the cord
itself is the most
frequent reason for
not attempting
direct repair on
these injuries.
However, most
patients requiring
surgical treatment
have (1) proximal
lesions close to
and, in some cases,
involving the spinal
cord, and (2)
lengthy lesions
requiring long
grafts for repair.
If the sensory root
has evidence of a
very proximal
(preganglionic)
injury, successful
repair of the motor
root at such a
proximal level is
technically
difficult, although
not impossible.
Direct repair is
impossible if the
roots are avulsed
from the spinal
cord, or if
secondary cord
damage makes
regeneration through
the grafts unlikely.
Some of these
patients may be
candidates for
neurotization
procedures.
Also
not candidates for
operation are
stretch lesions
confined to the
lower plexus
elements, such as C8
and T1 nerve roots,
or the lower trunk
to the medial cord.
Results with repair
of these lesions are
poor, except in
children. Adults
seen 1 year or later
after injury do not
usually benefit from
direct neural
repair, and thus are
not good operative
candidates for such
an approach.
Other
relative
contraindications
("stops") to surgery
include flail arm,
Horner's syndrome,
and meningoceles and
other myelographic
abnormalities (Table
2). Patients with
total arm paralysis
due to severe
brachial plexus
stretch injury are
very difficult to
salvage by direct
repair. It is
especially difficult
to recover distal
forearm and hand
function in patients
with flail arms. In
these patients,
every attempt is
made to regain
significant shoulder
abduction and
flexion of the
forearm. Presence of
Horner's syndrome,
although an
indication of
proximal T1 and/or
C8 root injury. does
not necessarily mean
that roots at higher
levels are damaged
at such a proximal
level.
|
TABLE 2
Selection
of
Plexus
Stretch
Injuries
for
Operation |
|
Clinical
questions: |
|
1. |
Is
lesion
complete
or
incomplete
in
distribution
of one
or more
elements?
|
|
2. |
Does
significant
motor
(not
sensory)
improvement
occur in
first 4
months? |
|
3. |
If
injury
is at a
nerve
root
level,
how
proximal? |
|
Relative
"stops"
(contraindications
to
direct
neural
repair): |
|
1. |
Winging
of the
scapula-long
thoracic
nerve |
|
2. |
Rhomboid
paralysis-dorsal
scapular
nerve |
|
3. |
Diaphragm
paralysis-phrenic
nerve |
|
4. |
Extensive
paraspinal
denervation
by
EMG-posterior
branch
of
anterior
root
(more
distal
and,
thus,
repairable
injury
to some
roots
may
still be
present)
|
|
5. |
Positive
sensory
potentials
can
suggest
preganglionic
injury
at ca,
T1, and
sometimes
C7;
higher
roots
may
still be
operable |
|
6. |
Myelopathy
and/or
fracture/dislocation
of spine |
|
Less
certain
"stops": |
|
1. |
Total
flail
arm |
|
2. |
Sensory
improvement
without
motor
improvement |
|
3. |
Horner's
syndrome |
|
4. |
Meningoceles
at some
(usually
lower)
but not
all
levels |
|
|
a. |
False
positive
and
negative
rates
are
significant.
|
|
|
b. |
Meningoceles
strongly
suggest
but do
not
prove
proximal
root
damage. |
|
|
c. |
Meningoceles
at one
or more
levels
suggest
but do
not
prove
proximal
damage
at other
root
levels
without
meningoceles. |
|
|
d. |
Absence
of
meningoceles
does not
prove
lateral
damage
nor does
presence
of a
meningocele
always
mean
proximal
damage
or any
damage
at all. |
 Informed Consent
Informed Consent
Once
the data derived
from the clinical
examination and the
electrophysiologic
and radiologic
studies are
assembled, the
managing physician
should sit down with
the patient and the
family to explain
the sites and nature
of the nerve
injuries. Most
patients will be
familiar with
electrical cables,
and this may serve
as a good analogy,
as long as they
understand that
restoration of
continuity does not,
alone, produce
function. It is
useful to explain to
the patient that the
delay following
injury is according
to plan, and has
allowed distinction
of those elements
which show evidence
of recovery from
those nerve elements
which do not.
The
patient and the
family should also
understand that the
patient will be
under the care of
the managing
physician for 2-6
years, during which
time spontaneous
recovery and
recovery following
surgery is observed
and further
management decisions
are made. By 2-3
years after repair,
sufficient return
will have occurred
so as to allow the
experienced
evaluator to give a
prognosis regarding
the ultimate
function. This sets
the stage for
appropriate
reconstructive
operations, such as
muscle transfers and
joint fusions, which
will further
facilitate
functional recovery
of the limb.
The
patient must
understand that a
personal quest for
recovery is an
absolute
prerequisite for a
successful outcome.
Initially the
patient may receive
direction from a
physical therapist,
but very rapidly the
patient should
receive the physical
therapy program at
home. Victims of
nerve injury should
be forewarned that
they may experience
uncomfortable
sensations during
the regeneration
phase, and that it
is essential for
them to be vigorous
in their exercise
program or these
uncomfortable
sensations will
assume an
overwhelmingly
negative context. If
the patient is not
prepared to actively
strive for recovery
with diligence for 2
years, there is
scarcely any reason
to attempt intricate
nerve surgery as the
patient's lack of
compliance will
ensure a poor
result.
The
experienced
clinician should,
relatively speaking,
be able to predict
the functional
status of the
patient several
years following
nerve repair.
Appropriate
vocational and
educational goals
may then be outlined
for the patient. If
it is immediately
apparent that an
individual will
never be able to
resume an occupation
involving heavy
physical labor,
appropriate
vocational
retraining should be
instituted at the
beginning rather
than at the end of
the management
program.
Patients with
devastating
neurologic injuries
may require
considerable
psychological
support initially.
This support should
be withdrawn by 6
months after the
repair. The patient
should be totally
independent in the
performance of
exercise programs,
visiting physical
therapy departments
with checks in
progress,
application of
splints, etc. The
coincidence of
severe head injury
with major
peripheral nerve
injury may carry a
relatively poor
prognosis. Patients
lacking in
motivation following
head injury will
usually not have the
drive to
successfully
complete the entire
course of
rehabilitation
during the period of
nerve regeneration.
 Technical
Considerations
Technical
Considerations
Peripheral
nerve
operations
should only
be performed
in operating
rooms with
appropriate
facilities
that include
excellent
illumination
and the
provision of
magnification
during
surgery. A
range of
instruments
is required
from
conventional
forceps and
scissors to
delicate
microinstruments.
An
appropriate
range of
sutures from
10-0 through
1-0 is
required.
Cases vary
in duration,
and the
duration of
the
operation is
often
unpredictable
as the final
decisions
for and
against
grafting are
only made
during the
operation
itself.
Because of
the
uncertainty
of the
nature of
the surgery,
these
operations
should be
undertaken
by surgeons
who have the
ability to
perform
external
neurolysis,
nerve
suture,
nerve
grafting,
and
neurotization
procedures.
Facility
with vein
and arterial
repair is
also
essential.
Operating
rooms must
have
appropriate
electrophysiologic
equipment
and
personnel so
that
intraoperative
NAP and SEP
studies can
be
repeatedly
conducted
during the
course of an
operation.
Failure to
achieve useful
regeneration to
the extent that
is expected
after nerve
repair may be
due in part to
excessive
conservatism on
the part of the
surgeon, who may
be reluctant to
resect scarred
nerve ends back
to normal nerve
segments when
this creates an
extensive gap to
be bridged.
Incisions for
exposure of the
lesion should be
large enough to
fully mobilize
the injured
neural
element(s)
(Figure 2).
Mobilization of
the nerve is
necessary to
overcome the gap
that results
from retraction
of the stumps
and resection of
the damaged
nerve ends.
Maximal nerve
length is
obtained by
dissecting to
and somewhat
beyond the
distal and the
proximal joints.
Neurophysiologic
studies have
shown that
extensive
mobilization
does not affect
the subsequent
function of a
normal nerve or
the regenerative
process in one
that is injured.
Mobilization
allows the
surgeon to
resect back to
healthy-appearing
neural tissue,
to shorten the
gap between
resected stumps,
and to join
separated nerve
ends.
Nonetheless,
grafts are a
useful
alternative and
most large gaps
can be closed by
a combination of
nerve
mobilization and
graft repair.
Repair of a
nerve will yield
the best results
when there is no
cross-sectional
area of scar to
block a maximal
down growth of
axons from above
or to prevent
the maximum
availability of
receiving
tubules
distally. The
appearance of
healthy nerve
ends usually
coincides with
brisk bleeding.
Healthy nerve
ends facilitate
maximum
fascicular
apposition when
the nerve ends
are brought
together
end-to-end or
are joined by
multiple
interfascicular
grafts. This
helps to promote
the entry of
regenerating
axons into
fasciculi of the
distal stump and
not into
interfascicular
epineural
tissue.
Distraction in
nerve repair
should be
avoided. This
makes
mobilization,
transposition,
and accurate
apposition of
the nerve stumps
all the more
important. If
the nerve is
under too much
tension,
distraction is
likely to occur,
particularly if
flexion of the
extremity is
needed to gain
an end-to-end
apposition. In
this case, the
surgeon should
resort to
relatively short
interfascicular
nerve grafts,
using an
autologous nerve
such as the
sural nerve. The
importance of
tension as a
contributing
factor for
failure of nerve
repair has been
demonstrated
experimentally
in the primate
model.
Other factors
leading to
failure of nerve
repair include
tissue
manipulation
that is not
gentle,
sacrifice of
longitudinal
vessels deep to
the epineural
level, and
intraoperative
or postoperative
stretch of the
nerve.
 Patient
Follow-up
Patient
Follow-up
Prior to
discharge from
hospital, the
patient should
have a very
clear
understanding of
what was
performed at
operation. A
simple sketch
may assist in
explaining.
Sutures and
staples are
conventionally
removed between
the eighth and
tenth
postoperative
day. Those
removing the
sutures should
understand the
required
duration of
immobilization.
Following simple
neurolysis, the
managing
physician may
want the patient
to move into a
vigorous passive
and active
physical therapy
program without
delay. If a
direct nerve
suture has been
performed,
immobilization
of the joints
may be required
for 3 or more
weeks. with
progressive
extension of the
joints for up to
6 weeks.
Premature
mobilization may
result in
suture-line
disruption.
Those with graft
repairs are
permitted more
motion in the
early weeks but
should avoid
hyperextension
or
hyperabduction
of the joints.
If possible, it
is appropriate
to reassess the
patient at
approximately 6
weeks following
nerve repair.
This allows the
physician to be
certain that the
patient
understands what
is required and
that the patient
is not adopting
an overly
passive stance.
Third-party
insurance
agencies should
understand the
significance of
the injury and
the likely
duration of
disability. If
the patient can
return to the
labor force,
even in a
reduced
capacity, this
should be
encouraged
immediately.
The next
follow-up visit
should coincide
with the
anticipated time
of initial motor
recovery of the
first downstream
muscle. At this
stage, the
patient should
be encouraged to
exercise those
muscles which
have received
axonal regrowth.
This is also a
good time to
observe whether
or not there is
evidence of
malingering.
medical-legal
neurosis, or
prolongation of
disability by
compensation
payments. In
these cases,
evidence of
sympathetic
dystrophy may
become more and
more obvious as
prolonged
overprotection
and
immobilization
of limbs leads
to secondary,
sympathetically
maintained pain
syndromes and
joint
contracture.
Decision-making
with regard to
patients
suffering from
peripheral nerve
injury is
straightforward
if there is
either clear-cut
evidence of
progressive
improvement or
obvious evidence
of no
improvement. The
management of
patients
demonstrating
partial
improvement
requires
considerable
experience. On
occasion,
re-exploration
may be judged
too risky, as
the patient has
too much to lose
and not enough
to gain. On
other occasions,
reoperation may
be indicated.
 |
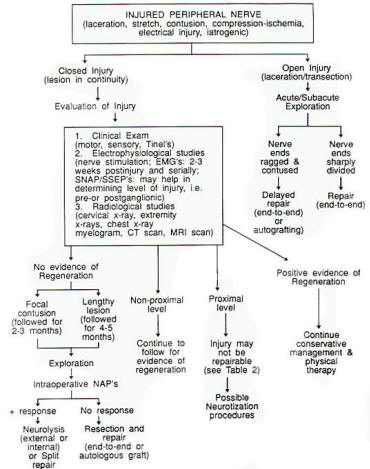 |
|
Fig-2:
Some
incisions
for
certain
nerves. |
Fig-3:
Flow
chart of
peripheral
nerve
injuries
management. |
|