|
Anamnesis:
 |
The patient,
K. H. Saraj,
a young man 30 years old, came to Jordan
from YAR 05-December-2009 complaining of fever
for one month duration with lethargy.
17-November-2009, when he was trying to travel
to Jordan, his condition deteriorated
dramatically and went to coma for what the
travel was post bonded. CT-scan and MRI
performed to him and several investigations were
performed. Lab investigations done for him:
HBc-Ab(IgG) positive 0.004 with toxoplasma
IgG (Automated ELISA) positive 918 IU/ml. These
data achieved 18-November-2009. WBC was 21.2
K/uL with neutrophils 92.4% (done
19-November-2009). WBC was 19.3 X10*9/L with
neutrophils 80% 20-november-2009. Toxo IgG
(Axsym) positive 26.9 IU/ml in 22-November-2009.
Toxo IgG (Axsym) positive 26.7 IU/ml in
24-November-2009. WBC 7.46 K/uL with
neutrophils 79.7%, GGT 38 IU/L recorded
26-November-2009. K+ was 2.8
mmol/L in 29-November-2009. K+
corrected to 3.4 mmol/L in 01-December-2009.
SGPT (ALT) was 66 IU/L and SGOT(AST) 40 IU/L in
02-December-2009. The patient was put in
mannitol and dexametasone pyrimethamine and
clindamycin. He was also covered with
antibiotics and aniviral and anti-tbc drugs. The
report provided with the patient confirming that
the patient was at admission comatose with
dilated both pupils and bradycardia
30/min. The patient slowly regained
consciousness and started to recover and was
oriented with normal vital signs before the
transfer. |
 |
The
patient was seen in the ward at Shmaisani
hospital. He had severe headache and inability
to walk with still signs of lethargy and
projectile vomiting with Foleys catheter. He was
responding to verbal commands and moving four
limbs with good power. There was no sensory
deficit with slight meningism. There was massive
rash all over the body and yellow skin and eyes.
|
 |
MRI of the brain was done
07-December-2009 and MRA of the brain with MRI
of the brain with contrast repeated
08-December-2009 showing the subependymar mass
in the region between the head of the right
caudate nucleus and the anterior commissure.
There was no aneurysm and there was still
enhancement of the ring around the mass, which
slightly shrunken in comparison to the MRI
performed 17-Novemeber-2009. |
 |
Lab investigations were
performed and HIV-1 & 2 antibody were negative.
Toxoplasma IgG was 17.5 IU/ml, SGOT 97.0 U/L,
SGPT 209.0 U/L LDH 545.0 U/L GGT 67.0 U/L Folate
serum level 10.0 NG/ml done 08-December-2009. |
 |
The patient was treated for
toxoplasmic encephalitis with abscess and the
patient projectile vomiting stopped and he could
walk the third post-admission day and the Foleys
catheter was removed 10-December-2009. He
admitted that the blurred vision disappeared. Chest and
abdomino-pelvic CT-scan done 11-December-2009
was normal. |
 |
Gradual tapering of dexametasone started
10-December-2009. Mannitol was stopped 11-December-2009.
CD4-Lymphocyte count 465 cell/uL and SPECT was performed by
Tl-201 13-December-2009 confirming no evidence of active
lesion in the brain and the patient was discharged
14-December-2009. |
 |
The patient,
then rapidly deteriorated in Yemen and
was admitted to the local hospital with severe
impairment of the level of consciousness with
right sided panophthalmoplegia and
aggressive treatment with mannitol, vancomycin,
antifungal and acyclovir was started. |
 |
MRI performed there was not informative
and the MRA was looking with beaded arteries. ANCA was
positive. By telephone communication it was suggested that
the patient has vasculitis. The patient was sent back to
Jordan and was admitted to the ICU of Shmaisani hospital.
The patient is no locked in syndrome with right sided plegia
and some times yawning. He had nuchal rigidity with high
fever. |
 |
The respiratory drive was compromised but
he was not in need for ventilation. MRI with contrast with
MRA and MRV done ruled out the presence of beaded arteries
and the presence of massive meningoencephalitis of the deep
basal ganglia both sides with involvement of the tentorium
and subtentorial structures. There was considerable
dilatation of the ventricular system. ANCA was repeated
twice and it was negative. The patient was started with the
same treatment as In Yemen and external drain was inserted
to the right lateral ventricle. The CSF was yellowish and
sent for all possible investigations. Only yeast could be
found in one specimen and was not found in others. The
pressure of the CSF was almost normal and mannitol was
stopped. All virology studies were normal and blood
for CXS was negative. Duflucan was increase and the
toxoplasmosis drugs were all the time kept. Meropnem
was started 1 gm QTD. |
 |
HIV tests were repeated and they were
negative. The patient was given petaglobin over three days
without any signs of improvement. |
 |
The external drain was removed after 7
days and 2 days later tapering the dexametasone was
started. The patient after slight improvement during the
first week started to show gradual deterioration 3 days
after tapering dexametasone, for what it was put back in
track. |
 |
The patient progressed signs of
decerebration with flexor spasms, for what senimet was given
and the next day Liorezal 5 mg was added. The patients
rigidity disappeared and the respiratory drive got worse,
for what Liorezal was stopped. |
 |
Two days later after stopping Liorezal
the patient showed slight improvement in the motor functions
and the rigidity disappeared, which means that he got severe
sensitivity to Liorezal. |
 |
MRI of the brain with contrast was
repeated 11-February-2010 which showed dramatic
deterioration of the lesion with involvement of the temporal
lobes more to the left and the pons. CSF was obtained
through the old ventricular catheter site and sent for
routine and antitbc PCR. The old investigated CSF for fungi
needs 45 days to identify the type of fungi, which could be
negative. |
|
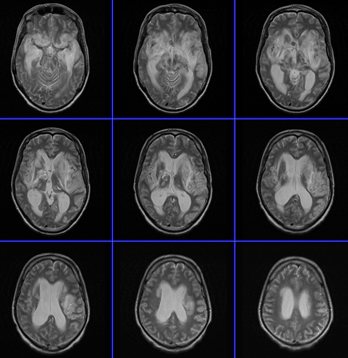
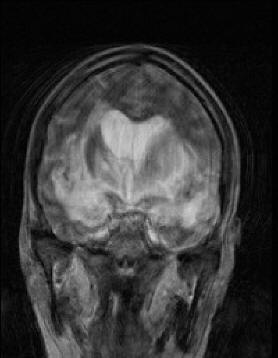 |
|
MRI performed 11-February-2010 showed
further deterioration despite aggressive treatment. |
 |
The patient in 12-February-2010 showing
slight improvement, and due to financial problems the
patient relatives decided to transfer him back to Yemen. |
 |
The patient has very aggressive
meningo-encephalitis of protracted and relapsing and
remitting character. Covering all the causative possible
cause, the morphologic changes got worse and worse. Could
toxoplasmosis can lead to such events, still a dilemma.
|
Toxoplasmosis
in HIV-infected patients
INTRODUCTION — Toxoplasmosis, an
infection with a worldwide distribution, is caused by the
intracellular protozoan parasite, Toxoplasma gondii. Immunocompetent
persons with primary infection are usually asymptomatic, but latent
infection can persist for the life of the host.
In immunosuppressed patients, especially patients with the acquired
immunodeficiency syndrome (AIDS), the parasite can reactivate and
cause disease, usually when the CD4 lymphocyte count falls below 100
cells/µL. All patients with human immunodeficiency virus (HIV)
infection should be screened for T. gondii antibodies. Counseling on
preventing toxoplasmosis should be given to those who are
seronegative and prophylaxis initiated, when appropriate, for
seropositive patients.
EPIDEMIOLOGY
Prevalence of infection — Seroprevalence rates of toxoplasmosis vary
substantially among different countries (eg, approximately 15
percent in the United States to more than 50 percent in certain
European countries). Among HIV-infected patients, seroprevalence of
antibodies to T. gondii mirror rates of seropositivity in the
general population. Among 2525 women in the United States, for
example, the T. gondii seroprevalence was 15 percent and did not
differ based upon whether or not the individual had HIV. Those with
HIV were more likely to have antibodies to T. gondii if they were 50
years of age or born outside of the United States. In patients with
AIDS, there is no higher incidence of toxoplasmosis in cat owners
compared to non-cat owners.
Patients with AIDS and <100 CD4 cells/µL, who are toxoplasma
seropositive, have an approximately 30 percent probability of
developing reactivated toxoplasmosis if they are not receiving
effective prophylaxis. The most common site of reactivation is the
central nervous system (CNS); toxoplasmosis is the most common
parasitic CNS opportunistic infection (OI) in AIDS patients , except
in patients who are on appropriate prophylaxis.
 |
Toxoplasmic encephalitis —
The incidence of toxoplasmic encephalitis in
AIDS patients reflects seropositivity rates in
this population; HIV-infected patients from
Florida, for example, had a higher
seroprevalence of T. gondii antibodies and a
greater prevalence of toxoplasmic encephalitis
in one study. However, the introduction of
anti-toxoplasma prophylaxis and highly active
antiretroviral therapy (HAART) has altered the
occurrence of toxoplasmic encephalitis like
other OIs. In the Multicenter AIDS Cohort Study
(MACS), the incidence of CNS toxoplasmosis
decreased from 5.4 per 1000 person-years in 1990
to 1992 to 3.8 per 1000 person-years in 1993 to
1995 and, 2.2 per 1000 person-years in 1996 to
1998 after HAART began to be widely used. |
 |
Extracerebral toxoplasmosis — It is much
harder to determine the incidence of extracerebral
toxoplasmosis. Most of the available data are from before
the introduction of HAART and from France where the
seroprevalence to T. gondii is high. In one French series of
1,699 HIV-infected patients, for example, the overall
incidence of toxoplasmosis was 1.53 cases per 100
person-years for the years 1988 to 1995 (compared to 5.4 or
3.8 cases per 1000 person-years in the United States). The
distribution of extracerebral cases is illustrated by the
following: In the series of 1,699 patients, 116 cases of
confirmed, probable or possible toxoplasmosis was diagnosed;
cerebral toxoplasmosis accounted for 89 percent, with
pulmonary, ocular, and disseminated infection responsible
for 6, 3.5, and 1.7 percent of cases, respectively. Another
French series of 199 HIV-infected patients with
extracerebral toxoplasmosis evaluated between 1990 and 1992
estimated that the prevalence of extracerebral toxoplasmosis
among AIDS patients was 1.5 to 2 percent. Among these
selected patients, eye involvement occurred in 50 percent,
lungs in 26 percent, two or more extracerebral sites in 11.5
percent, and peripheral blood, heart, and bone marrow in 3
percent each. Involvement of the bladder, pharynx, skin,
liver, lymph nodes, conus medullaris, and pericardium also
were demonstrated in rare cases. Widespread distribution may
be more common pathologically than clinically appreciated.
This was suggested in an autopsy study in which the most
common extracerebral sites of toxoplasmosis in HIV-infected
patients were the heart, lungs, and pancreas with 91, 61,
and 26 percent of cases, respectively.
The most prominent risk factor for the development of
extracerebral toxoplasmosis is advanced immunosuppression
(mean CD4 counts of 57 and 58 cells/µL in two of the French
series). Concurrent CNS disease was present in 41 percent of
patients in the report of 199 extracerebral cases.
|
CLINICAL PRESENTATION
— As noted above, when T. gondii reactivates in a patient with AIDS,
it most commonly does so in the CNS leading to cerebral abscesses.
The clinical manifestations of toxoplasmosis in immunocompetent
patients are discussed separately.
 |
Toxoplasmic encephalitis —
Patients with cerebral toxoplasmosis typically
present with headache. In one retrospective
review of 115 cases, 55, 52, and 47 percent had
headache, confusion, and fever, respectively.
Focal neurologic deficits or seizures are also
common. Fever is usually, but not reliably,
present. Dull affect may be due to global
encephalitis but more profound mental status
changes, especially accompanied by nausea or
vomiting, usually indicates elevated
intracranial pressure. |
Extracerebral toxoplasmosis
 |
Pneumonitis — Pneumonitis, as
an extracerebral manifestation of toxoplasmosis,
presents with fever, dyspnea and non-productive
cough. Chest radiographs typically have
reticulonodular infiltrates. The clinical
picture may be indistinguishable from
Pneumocystis jiroveci (formerly carinii)
pneumonia (PCP). Some have suggested that a
blood level of lactate dehydrogenase >600 U/L is
more likely to be associated with toxoplasmosis
than PCP. However, others have found that LDH is
elevated in both of these infections.
|
 |
Chorioretinitis — Patients with
toxoplasmic chorioretinitis (a posterior uveitis) usually
present with eye pain and decreased visual acuity. These
symptoms and signs do not distinguish this entity from other
ocular infections in HIV, especially cytomegalovirus (CMV)
retinitis. Toxoplasmic chorioretinitis appears as raised
yellow-white, cottony lesions in a non-vascular
distribution, unlike the perivascular exudates of CMV
retinitis. Vitreal inflammation is usually present in
contrast to ocular toxoplasmosis in immunocompetent
patients. Chorioretinitis due to T. gondii can rarely mimic
acute retinal necrosis. Up to 63 percent of AIDS patients
with toxoplasma chorioretinitis will have concurrent CNS
lesions. |
 |
Other manifestations — Toxoplasmosis can
rarely present with involvement of a variety of other sites
in patients with AIDS including: gastrointestinal tract,
liver, musculoskeletal system, heart, bone marrow, bladder,
spinal cord, and orchitis. In the French series,
toxoplasmosis of the pancreas or muscular system did not
occur in the absence of involvement at other sites. Nine
patients with disseminated toxoplasmosis presenting with
septic shock were reported from France; in two patients this
appeared to be primary infection. |
DIAGNOSIS
— Toxoplasmosis frequently enters the differential diagnosis in an
AIDS patient with focal brain lesions. The vast majority of patients
with toxoplasma encephalitis are seropositive for anti-toxoplasma
IgG antibodies. Anti-toxoplasma IgM antibodies are usually absent
and quantitative IgG antibody titers are not helpful. The absence of
antibodies to toxoplasma makes the diagnosis less likely, but does
not exclude the possibility of TE.
To establish a definitive diagnosis in patients with extracerebral
toxoplasmosis, the demonstration of tachyzoites in tissue or fluid,
such as BAL, is usually required.
Cerebral toxoplasmosis — Most AIDS patients with cerebral
toxoplasmosis have multiple, ring-enhancing brain lesions often
associated with edema. In a report of 45 patients who underwent
computed tomography (CT) or magnetic resonance imaging (MRI), 31 (69
percent) had multiple lesions and 14 had single lesions. There is a
predilection for involvement of the basal ganglia.
MRI versus CT — Magnetic resonance imaging (MRI) is more sensitive
than computed tomography (CT) for identifying these lesions. In one
prospective study of 50 AIDS patients with neurologic symptoms, for
example, MRI detected abnormalities that influenced diagnosis and
treatment in 40 percent, which were not characterized by CT.
However, there is an extensive differential diagnosis for brain
lesions in AIDS patients and the appearance by either CT or MRI does
not adequately distinguish among these. Toxoplasmosis and CNS
lymphoma are the two most common entities (representing 50 and 30
percent, respectively), but other infections, including
cryptococcosis, histoplasmosis, aspergillosis, tuberculosis and
trypanosomiasis may also cause brain abscesses in patients with
AIDS.
SPECT imaging — Thallium single photon emission computed
tomography (SPECT) and positron emission tomography (PET) can be
useful in distinguishing toxoplasmosis or other infections from CNS
lymphoma. Lymphoma has greater thallium uptake on SPECT and greater
glucose and methionine metabolism on PET than neurotoxoplasmosis or
other infections.
Brain biopsy — Definitive diagnosis of cerebral toxoplasmosis is
made by pathologic examination of brain tissue obtained by open or
stereotactic brain biopsy. Organisms are demonstrated on hematoxylin
and eosin stains; some laboratories also use immunoperoxidase
staining which may increase diagnostic sensitivity.
However, there is morbidity and even mortality associated with the
procedure. In one series of 136 patients, morbidity and mortality of
brain biopsy was 12 and 2 percent, respectively. Morbidity rates
have been reported to be 3 to 4 percent. Due to these concerns, it
is common practice to presumptively diagnose and treat cerebral
toxoplasmosis in AIDS patients if the clinical suspicion is high. A
presumptive diagnosis can be made if the patient has a CD4 cell
count <100/µL and: Is seropositive for T. gondii IgG antibody Has
not been receiving effective prophylaxis for toxoplasma. Brain
imaging demonstrates a typical radiographic appearance (eg, multiple
ring-enhancing lesions)
If these three criteria are present, there is a 90 percent
probability that the diagnosis is toxoplasmosis, and thus, it is
common practice to treat empirically for toxoplasmosis. If a
solitary lesion is detected, even if toxoplasma serology is
positive, CNS lymphoma rises on the differential diagnosis list. If
all three of the above criteria are not met, biopsy or other
diagnostic tests should be performed. Brain biopsy should also be
performed if the patient does not respond to empiric therapy, on the
basis on clinical or radiographic improvement.
A group has questioned the reliability of the presumptive
diagnostic approach in favor of earlier biopsy. In one study, the
positive predictive value of the Centers for Disease Control and
Prevention (CDC) criteria for the diagnosis of toxoplasmic
encephalitis declined from 100 to 39 percent from 1991 to 1996. An
increase in use of prophylaxis against toxoplasmosis and increases
in other causes of CNS focal lesions were deemed largely responsible
for this difference.
Polymerase chain reaction (PCR) testing for other pathogens (eg,
Epstein-Barr virus [EBV], JC virus, Mycobacterium tuberculosis,
Cryptococcus neoformans) can be considered in patients with focal
brain lesions who were already taking prophylactic antibiotics for
toxoplasmosis or were seronegative. The prevalence of these other
infections in HIV-infected patients and other clinical clues from
the presentation should influence which specific tests are ordered.
CSF analysis — Cerebrospinal fluid (CSF) may have mild mononuclear
pleocytosis and elevated protein. T. gondii can be detected in CSF
by DNA amplification in most patients with CNS infection.
Tachyzoites can sometimes be seen on cytocentrifuged cerebrospinal
fluid samples stained with Giemsa.
TREATMENT — A number of
therapies are available for the treatment of toxoplasmosis.
Toxoplasmic encephalitis generally responds promptly to treatment.
Lack of either clinical or radiographic improvement within 10 to 14
days of empiric therapy for toxoplasmosis should raise the
possibility of an alternative diagnosis. Extracerebral toxoplasmosis
is treated with the same regimens as toxoplasmic encephalitis,
although the response may not be as favorable.
The following are treatment guidelines from the Centers for Disease
Control and Prevention (CDC), National Institutes of Health (NIH,
and Infectious Diseases Society of America (IDSA):
First-line therapy — Two combination regimens are considered to be
first choices for the treatment of toxoplasmosis. All pyrimethamine
regimens should include folinic acid to prevent drug-induced
hematologic toxicity (10 to 25 mg/day PO).
Pyrimethamine (200 mg
loading dose PO followed by 75 mg/day) plus
sulfadiazine (6 to 8
g/day PO in four divided doses). For those patients who cannot take
sulfadiazine due to intolerance or history of allergy, pyrimethamine
(200 mg loading dose PO followed by 75 mg/day) plus clindamycin (600
to 1200 mg IV or 450 mg PO four times a day) is recommended.
Pyrimethamine plus sulfadiazine has a higher incidence of cutaneous
hypersensitivity reactions compared with pyrimethamine plus
clindamycin but may have a lower incidence of relapse. Patients
receiving sulfadiazine therapy do not require additional TMP-SMX for
PCP prophylaxis. One study documented equivalent pharmacokinetic
parameters for 2000 mg of sulfadiazine administered twice daily
compared to 1000 mg given four times a day. Treatment for pregnant
females is the same as in nonpregnant females.
Alternative regimens — Several alternative regimens have been used,
generally in patients who are unable to tolerate either sulfadiazine
or clindamycin: Pyrimethamine (200 mg loading dose PO followed by 75
mg/day) plus azithromycin (1200 to 1500 mg PO once daily)
Pyrimethamine (200 mg loading dose PO followed by 75 mg/day) plus
atovaquone (750 mg PO four times a day) Sulfadiazine (1500 mg four
times a day) plus atovaquone (1500 mg twice daily)
If atovaquone is used, measuring plasma levels might be helpful
since there is significant individual variation in drug absorption
and higher plasma concentrations are associated with better
outcomes.
A pilot, multicenter, randomized prospective trial evaluated the
efficacy and safety of trimethoprim (TMP) and sulfamethoxazole (SMX)
compared to standard therapy with pyrimethamine-sulfadiazine.
Seventy-seven patients were randomly assigned to receive TMP (10
mg/kg/day) and SMX (50 mg/kg/day) as acute therapy for four weeks
followed by maintenance therapy for three months at half of the
original dosage. There was no statistically significant difference
in clinical efficacy between the two arms. Adverse effects were more
common in the patients treated with pyrimethamine-sulfadiazine.
Trimethoprim-sulfamethoxazole may be an effective alternative
treatment regimen, particularly in resource-poor settings.
In critically ill patients, intravenous administration of TMP 10
mg/kg/d + SMX 50 mg/kg/d, can be considered, although is less
effective.
As above, pyrimethamine therapy should always be accompanied by the
administration of folinic acid (10 to 25 mg/day PO). Some of these
regimens, especially those using atovaquone, have been tried as
salvage therapy in patients failing to respond to a first-line
regimen. However, reconsideration of the diagnosis should be the
first response to a patient who appears to be failing therapy.
Duration of therapy — For patients who respond, the duration of
therapy is typically six weeks at the doses recommended above.
Following that treatment, it is usually safe to decrease to a lower
dose for secondary prophylaxis (chronic suppressive therapy).
Steroids — Adjunctive corticosteroids should be used for patients
with radiographic evidence of midline shift, signs of critically
elevated intracranial pressure or clinical deterioration within the
first 48 hours of therapy. Dexamethasone (4 mg every six hours) is
usually chosen and is generally tapered over several days.
When corticosteroids are used, it may be difficult to assess the
clinical response to antibiotics since the rapid improvement in
symptoms may be due to steroid therapy. Radiographic assessment is
also affected since the corticosteroids will reduce the intensity of
ring-enhancement and the amount of surrounding edema. If steroids
are used, patients should also be carefully monitored for the
development of other opportunistic infections.
Anticonvulsants — Anticonvulsants should be administered to patients
with a history of seizures, but should not be given routinely for
prophylaxis to all patients with the presumed diagnosis of TE.
Careful attention needs to be paid to any potential drug
interactions.
Monitoring of therapy — Monitoring of
patients includes careful clinical evaluations, serial brain
imaging, and assessment of any adverse effects of therapy. There is
no value to serial assessment of IgG toxoplasma antibody titers.
Common side effects of pyrimethamine include rash, nausea, and bone
marrow suppression. Higher doses of leucovorin up to 50 to 100 mg
daily can be considered for management of hematologic abnormalities.
Sulfadiazine use can lead to rash, fever, leukopenia, hepatitis,
nausea, vomiting, diarrhea, and crystalluria. Clindamycin use can
also lead to fever, rash, nausea and diarrhea related to production
of Clostridium difficile toxin.
Response to therapy — Clinical
improvement usually precedes radiographic improvement. Thus, a
careful daily neurologic examination is more important than
radiographic studies in assessing the response to therapy during the
first two weeks of treatment. Radiographic reassessment should be
deferred for two to three weeks unless the patient has not
demonstrated clinical improvement within the first week or has shown
any worsening.
The literature on response to therapy is hampered by presumptive
diagnoses, cross-over treatments, and discontinuation for toxicity
rather than lack of clinical response. There are no randomized,
double-blind trials. The comparative trials on different treatment
regimens suggest that approximately 80 percent of patients
demonstrate clinical and radiologic responses.
One study focused on the timing of the response in 49 patients
treated with pyrimethamine plus clindamycin [22]. Seventy-one
percent responded overall with 32 of the 35 patients demonstrating
improvement of at least 50 percent of baseline abnormalities by day
14 of therapy. The authors concluded that early neurologic
deterioration or lack of neurologic improvement (except for headache
and seizures) by day 10 to 14 should raise the possibility of an
alternative diagnosis and such patients should be considered for
brain biopsy. (See "Approach to HIV-infected patients with central
nervous system lesions").
PROPHYLAXIS — Patients
seropositive for T. gondii should receive prophylaxis according to
the guidelines of the Centers for Disease Control and Prevention
(CDC), United States Public Health Service (USPHS) and the
Infectious Diseases Society of America (IDSA).
Primary prophylaxis — Prophylaxis is
indicated for patients with HIV and CD4 counts <100 cells/µL who are
T. gondii IgG positive. Patients who have negative toxoplasma
serology should be counseled to avoid eating undercooked meats and
to use gloves when carefully cleaning cat litter boxes. They do not
need to avoid contact with household cats entirely.
Secondary prophylaxis — As noted above
in the treatment section, following six weeks of therapy, patients
can receive lower doses of drugs which is considered secondary
prophylaxis or chronic suppressive therapy.
Sulfadiazine (2 to 4 g daily in 4 divided doses) plus pyrimethamine
(25 to 50 mg daily) is the first choice for secondary prophylaxis.
Folinic acid (10 mg to 25 mg daily) is also given concurrently.
Alternative regimens include: Clindamycin (300 mg PO QID or 450 mg
PO TID) plus pyrimethamine (25 to 50 mg daily) plus folinic acid (10
to 25 mg daily. Atovaquone 750 mg PO 2 to 4 times daily with or
without pyrimethamine 25 mg PO daily plus folinic acid 10 mg PO
daily.
Atovaquone monotherapy 750 mg four times daily can be considered for
patients who cannot tolerate pyrimethamine but the one year relapse
rate is 26 percent.
Toxoplasmosis prophylaxis and immune
reconstitution — If the CD4 count rises above 200
cells/µL for three months, primary prophylaxis for both PCP and
toxoplasmosis can be safely discontinued.
The issue of secondary prophylaxis is more complex. Patients appear
to be at low risk for recurrence of TE if they have completed
therapy, remain asymptomatic, and have a sustained increase in their
CD4+ T lymphocyte counts greater than 200 cells for more than six
months. The CDC/USPHS/IDSA guidelines state that if immune
reconstitution is maintained for six months, then secondary
prophylaxis for TE can be discontinued.
The clinician needs to remember that the number of patients who have
been evaluated with this approach is limited and the patient should
be educated about symptoms that should lead to a prompt medical
evaluation. Primary or secondary prophylaxis should be re-initiated
if the CD4+ T lymphocyte count declines to less than 200
cells/microL.
SUMMARY Seroprevalence
to T. gondii depends upon the part of the world in which the patient
was born and resided more than upon the patient's HIV status.
Toxoplasma encephalitis is the most common presentation of
toxoplasmosis as an OI among AIDS patients and occurs most commonly
in those with a CD4 count <100 cells/µL. Prophylaxis against PCP and
the widespread use of HAART in developed countries has greatly
decreased the incidence of this infection. The diagnosis of
toxoplasma encephalitis is usually made presumptively in an AIDS
patient with CD4 count <100/µL, a positive T. gondii IgG antibody,
no recent prophylaxis against toxoplasmosis, and multiple
ring-enhancing lesions on brain imaging. Brain biopsy can yield the
diagnosis in patients who don't meet the above criteria or whose
lesions fail to respond to presumptive therapy. First-line therapies
for cerebral toxoplasmosis include pyrimethamine plus sulfadiazine
or pyrimethamine plus clindamycin. Alternatives include
pyrimethamine plus azithromycin, pyrimethamine plus atovaquone,
sulfadiazine plus atovaquone, or as a last resort TMP/SMX. Folinic
acid must always accompany pyrimethamine therapy. Corticosteroids
are often administered in conjunction with antibiotics in patients
with signs of significant increased intracranial pressure. The doses
of pyrimethamine/sulfadiazine or pyrimethamine/clindamycin are
usually lowered for maintenance treatment after approximately six
weeks of therapy. Primary prophylaxis against toxoplasmosis is
usually given to patients with CD4 counts <200 cell/µL. TMP-SMX in
the doses given for PCP prophylaxis is the usual choice to
accomplish prevention of both OIs. Both primary and secondary
prophylaxis (maintenance therapy) can be discontinued in patients
who achieve immune reconstitution with HAART.
Comments
 |
The Toxo IgM (Axsym) was all
the time negative in this case. The patient had
no HIV. |
 |
The causative cause of his
rapid deterioration was the escalation of rapid
increase in ICP, because the abscess was near
the foramen of Monroe. |
|