|
 Indications for Operation
Indications for Operation
Microvascular decompression is indicated
in patients who have trigeminal neuralgia intractable to medical therapy
and for whom anesthesia does not pose an excessive risk. Elderly
patients are eligible if healthy and active. Prior destructive
procedures are not a contraindication to operation, but true anesthesia
dolorosa will not be relieved by microvascular decompression. A series
reported by Barba and Alksne showed that patients who have recurrent
trigeminal neuralgia following a destructive procedure have only a 43
percent rate of pain relief after microvascular decompression. Selective
sectioning of the portio major may be indicated in addition to the
microvascular decompression in this group of patients. However, the
results obtained in such patients have been almost as good as in virgin
cases. Most of patients have failed on drug therapy or had toxic
reactions. Several have not been given carbamazepine because of the fear
of side effects and toxic reactions by the referring physician and/or
the patients.
 Preoperative Evaluation
Preoperative Evaluation
Magnetic resonance imaging (MRI) without
and with contrast enhancement is performed to evaluate the presence of
ectatic arteries and to rule out extra-axial cerebellopontine angle
tumors and arteriovenous malformations. Computed tomography was used in
most cases before we began using MRI- again, without and with contrast
enhancement. Most patients coming with a prior study in hand. In these
patients, a repeat study or the other type of imaging study is performed
only if needed. Ectatic arteries that are present are not necessarily
the cause of the trigeminal neuralgia. Tumors usually cause trigeminal
neuralgia by vascular compression of the nerve. Small tumors, especially
meningiomas, may be missed with even the best imaging studies. Otologic
testing, including pure tone and speech discrimination testing as well
as assessment of brain stem auditory evoked potentials are part of
preoperative investigation.. Trigeminal evoked potentials and
electromyography of the temporal is and masseter muscles are frequently
obtained. An internal medicine consultation is obtained on older
patients. Other routine and special studies and consultations are
obtained as needed.
 Anesthetic Management and Monitoring
Anesthetic Management and Monitoring
The patient is placed on the operating
table with the head at the foot of the table. This allows the surgeon
enough knee room when operating in the sitting position. The patient is
anesthetized with sodium thiopental, intubated, and maintained with
nitrous oxide and oxygen. Intravenous fentanyl is given intermittently.
Paralyzing agents are used as needed. Intraoperative monitoring of brain
stem auditory evoked potentials is essential in decreasing the incidence
of hearing loss due to errors of retraction. Doppler ultrasound
monitoring over the sternum is instituted after the patient is placed in
the lateral position. The Doppler device is used primarily as a
convenient auditory pulse monitor for the surgeon, as air embolism is
practically nonexistent in most series of patients operated upon in the
lateral position.
 Operative Technique
Operative Technique
A three-point fixation
headholder is applied to the head, using local anesthetic infiltration
in the pin sites before tightening the pins. The patient is then turned to the contralateral
lateral decubitus position, and the connections of the headholder to
the table are loosely joined. A soft blanket roll is placed under the
axilla. The patient is taped and strapped across the hip to the table to
allow safe lateral rotation of the table if needed for intracranial
exposure. The neck is placed in traction with slight flexion and
ipsilateral rotation, and the connectors of the headholder are
tightened. It is important that the head not be brought forward away
from the surgeon and that there be no ipsilateral flexion. The
ipsilateral shoulder is taped in a caudal direction and forward as
necessary. The end result should be that the surgeon has free access to
the ipsilateral occipital boss
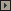 Incision
and Craniectomy
Incision
and Craniectomy
The hair on
the ipsilateral side behind the ear is shaved. The area is prepared for operation. The incision is marked by
placing a finger behind the mastoid eminence and constructing a line
behind the finger that generally parallels the hairline, extending
caudally for 3 to 6 cm from the level of the top of the pinna (the
incision should be longer in obese or heavily muscled patients). The
incision is carried down to the bone rostrally and through the galea
aponeurotica caudally. Bipolar electrocoagulation is used for
hemostasis as needed. The upper part of the incision is separated from
the bone with a large periosteal elevator. A small self-retaining
angulated or straight Weitlaner retractor with or without posts is put into place and tightened. Further
dissection is carried down to the occipital plate using bovie cutting
current and periosteal elevators. The Weitlaner retractor is tightened
gradually. Further hemostasis is achieved with electrocoagulation.
Emissary veins are sealed with bone wax. The posteromedial mastoid
eminence is cleared of soft tissue. The occipital plate is perforated.
the dura mater is separated, and a small superolateral retromastoid
craniectomy. about 2.5 cm in diameter, is made. Raney posterior fossa
rongeurs and Leksell rongeurs are most useful in making this
craniectomy. The craniectomy should extend to the sigmoid sinus even if
mastoid air cells are entered. The mastoid air cells of dolichocephalic
patients usually extend behind the sigmoid sinus. The edge of the
lateral sinus should be identified in all patients and partly exposed in
younger patients. This is not as important in women over the age of 50
and men over the age of 60 because the cerebellum is smaller in these
patients. The craniectomy should extend caudally behind the lateral
aspect. The bone edges are heavily waxed. If mastoid air cells are
entered, they are packed with muscle and bone wax.
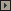 Dural
Opening and Early Exposure
Dural
Opening and Early Exposure
The dura mater is opened obliquely and incised back to the junction of
the lateral and sigmoid sinuses or incised in curvilinear fashion. The
resulting dural flaps are sewn back out of the way. The lateral sinus,
if exposed, can actually be tented up out of the way, exposing the
underside of the tentorium cerebelli and thereby facilitating exposure. In younger patients, the cerebellum may bulge out of the
opening. Should this occur, the cerebellum is greatly compressed back intradurally over a rubber dam and cottonoid and then elevated gently
off the floor of the posterior fossa. The subarachnoid cisterns over the
glossopharyngeal and vagus nerves are opened sharply. A microsurgical
retractor with a narrow rectangular or tapered blade is connected by two
rods and three connectors to the lower posterior post of the Weitlaner
retractor base. Alternatively. a gooseneck retractor may be used,
although retraction placement is not quite as precise. A 0.5 x 1.5 in. cottonoid strip is prepared with a slightly larger piece of rubber dam
under it. This is placed superolaterally over the cerebellum. The
retractor blade is bent proximally, usually to about 30 to 35 degrees
(the angle depends on the c0l!figuration of the patient). It is most
important that the blade have no curve in it other than the proximal
bend: otherwise it will interfere with the surgeon's line of vision
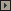 Microsurgical Exposure of the Trigeminal Nerve
Microsurgical Exposure of the Trigeminal Nerve
At this point. with the operating surgeon
sitting on a low stool, the microscope is brought into the field. The
tip of the retractor blade is placed high and lateral and the tentorium
visualized. The surgeon must be aware that bridging veins extending
posteromedially or out over the superior cerebellar surface may be
present, especially if the superior petrosal vein is small or absent.
If a vein is tom, it is better to treat it at once with bipolar
coagulation and division than to accept the bleeding or try to stop it
with packing. The junction between the tentorium and the petrous bone is
identified, and the cerebellum is retracted gently.
The retractor is placed superolaterally
over the cerebellum. Care is taken not to retract medially any more than
is necessary, as this direction of retraction may stretch the auditory
and facial nerves. The auditory nerve is identified as early as possible
in the dissection. and the arachnoid cisterns are opened rostral to it.
The superior petrosal vein. if present. crosses this arachnoid and
commonly has an inverted Y configuration. However. it may be short,
long. single. multiple. or absent. If absent. the surgeon should beware
of other large bridging veins in this area. One branch of
the vein at a time is coagulated. partly divided, and recoagulated. and
a Valsalva manoeuvre is then performed. If there is no bleeding. the vein
is again coagulated and divided, and the Valsalva manoeuvre again
performed. If bridging veins tear, they usually do so at a dural sinus.
They should be coagulated and divided and the distal end packed at the
sinus while satisfactory occlusion of the proximal end is ensured. At
this point, the sinus end can be coagulated again. and if that is
unsuccessful in stopping bleeding. it can be treated with a piece of
Gelfoam or Surgicel and pressure with a small cottonoid. This manoeuvre
will usually stop the bleeding. If bleeding is not easily controlled, the head of the operating
table is raised (reverse Trendelenburg manoeuvre) to the point where the
veins begin to collapse.
simplifying hemostasis. The surgeon must be aware that the
superior petrosal vein or a branch thereof may be the cause of the
trigeminal neuralgia. Therefore, the neurovascular relationships of this
vein and the trigeminal nerve should be clarified before the vein is
coagulated and divided, if at all possible.
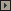 Evaluation of the Neurovascular
Compression
Evaluation of the Neurovascular
Compression
The
arachnoid over the trigeminal nerve is opened sharply. The tip of the
retractor blade is placed over the ala of the cerebellum, and the ala,
which normally covers the rostrolateral root entry zone of the
trigeminal nerve, is elevated posteriorly and caudally. If one is to
make valid observations and treat all possible sources of vascular
compression, the entire trigeminal nerve must be visualized from the brain stem to the dura.
Subtleties abound in the evaluation of neurovascular compression, and
the following rules regarding these observations may facilitate
appreciation of the pathologic changes:
1. Generally, lower facial pain (V3, V2-3)
is caused by blood vessels on the rostroanterior aspect of
the root entry zone. V1 pain is caused by a vessel on the caudal aspect
of the root entry zone. Isolated V2 pain is caused by a vessel on the
side of the nerve, most frequently a vein, which is often quite distal
on the nerve. This condition is more common in women.
2. Multiple vascular impingement is
common. All vascular compression should be treated.
3. The underside of the ala of
the cerebellum must be evaluated in all patients, as a blood vessel
located there may cause compression of the root entry zone.
4. The junctional area of central and
peripheral myelin extends out a considerable distance in the portio
major. Significant vascular compression of the nerve may therefore occur
close to or even at Meckel's cave.
5. Small pontine surface veins in the pia
may cause or contribute to trigeminal neuralgia.
6. When the patient is in the
contralateral lateral decubitus position, the offending blood vessel
may not be in direct contact with the trigeminal nerve. This occurs
because the brain stem and blood vessels move differentially with
changes in position of the patient.
7. Small veins running between fascicles
of the nerve may cause or contribute to compression and must be
coagulated and divided.
8. Small arterioles or venules may cause
or contribute to trigeminal neuralgia. The outside of an arteriolar
loop is enough to cause tic.
9. Mobilization of the significant blood
vessel or the placement of an implant at its location may be accompanied
by significant but brief bradycardia. This is the second best monitor
of efficacy- the best being the eyes and brain of the surgeon.
10. Vascular compression of the motor-proprioceptive
fascicles of the nerve distal to the pons causes constant pain. usually
centered in V 2 and usually burning in character.
Technique
of Vascular Mobilization and Decompression. The
pia-arachnoidal chordae over the trigeminal nerve should be cut sharply.
If the superior cerebellar artery (SCA) is causing compression. it will
often be found to have bifurcated (occasionally trifurcated) before or
at the site of compression. The entry zone of the portio minor must be
seen clearly in its entirety. The underside of the cerebellar ala should
be inspected. The entire course of the nerve to Meckel's cave must be
seen. The arterial loops are mobilized using microsurgical dissectors.
not by pinching with forceps. and are brought into a horizontal position
over the trigeminal nerve that the offending loops are resting easily
in their new attitude rostral to the nerve. Shredded Teflon felt is
moistened. rolled into various-sized pledgets. and placed under the
artery. The surgeon must make sure ( 1 ) that enough felt is placed to
separate the entire artery from the entire nerve: (2) that the felt
extends around the nerve and pons so that it cannot slip: (3) that it is
thick enough to pad the nerve satisfactorily. and (4) that it does not
kink or compress the artery. (These general rules are
the same for all arterial decompressions.) Horseshoe-shaped arterial
loops should have a tail of the implant fulled through the loop to
anchor it in place.
Veins bridging from the brain to the dura
are generally easy to separate from the nerve with sharp and blunt
dissection. Similarly, the implant is easy to place between the
trigeminal nerve and large bridging veins, or the veins may be
coagulated and divided instead. Small veins running through the nerve
should be coagulated and divided at multiple sites, necessitating
manipulation of the nerve. The lowest possible bipolar current should be
used to avoid spread of current to the trigeminal nerve. Small veins on
and around the nerve that lie in the pia should be coagulated, divided,
and excised if possible. These surface veins have a great tendency to
recanalize, and at present that is the most common cause of early
recurrence of neuralgia in most series. The recurrence occurs most
commonly 4 to 6 months postoperatively.
All blood vessels anywhere near the nerve
should be separated from it. The retractor is gently released to ensure
that relationships are stable and that blood vessels do not kink. Small
bits of felt are placed under the ala of the cerebellum if vessels are
present. Gelfoam is placed around the vein and implant if necessary to
ensure stability. The Valsalva manoeuvre is performed several times to
ensure hemostasis in the venous system.
 Points of Difficulty
Points of Difficulty
One difficulty arises when the whole nerve
cannot be adequately exposed. Usually the problem involves the root
entry zone, but occasionally the distal portio major is the site of the
problem. The distal nerve is harder to visualize; indeed, the entire
exposure is more difficult in dolichocephalic than in brachycephalic
patients, as the cranial nerves travel more laterally to their foramina
in the latter.
A second point of difficulty is that an
elongated arterial loop, especially a loop of the superior cerebellar
artery, cannot be easily brought out of the axilla of the nerve into a
horizontal position. This manoeuvre can almost always be accomplished by
starting distally on the artery (proximally on the nerve) and placing a
temporary piece of Teflon felt between the distal arterial loop and the
trigeminal nerve while the more proximal parts of the artery are
mobilized. More felt is placed distally, and the manoeuvre is repeated
as often as necessary. Perforating arterioles of the SCA are always
elongated and emerge from the rostral part of the loops. They move as
the loops are mobilized.
The next point of difficulty has to do
with small veins and arteries, especially the former. Small veins in the
pia and arachnoid and arterioles with the outside of a loop compressing
the nerve must be treated, as they may be the cause of the trigeminal
neuralgia. The veins must be lifted up before they are coagulated so
that current is not transmitted to the nerve. The veins tend to
recanalize, as noted above. Small arteries are easily decompressed and
held away with wisps of shredded Teflon felt.
 Closure
Closure
The dura
mater is closed in watertight fashion. A narrow strip of Gelfoam may be
placed under the suture line before sewing if necessary. A piece of
Gelfoam is placed over the dura, and a methylmethacrylate cranioplasty
is performed. Use of a cranioplasty has decreased the incidence of
chronic postoperative headaches ("pseudo-occipital neuralgia") to the
vanishing point. Also, patients prefer not to have a soft spot or
depressed area. The caudal deeper muscle layers are approximated after
the self-retaining retractor is removed. The remainder of the wound is
closed, and a dry dressing applied.
 Postoperative Care
Postoperative Care
In the recovery room, hypertensive
episodes are no more common after cranial nerve microvascular
decompression than after any other intracranial procedure. The blood
pressure should be controlled. The patient stays overnight in the
neurosurgical intensive care unit and returns to a standard hospital
room the next day. Headache is usually mild and brief if only a small
volume of cerebrospinal fluid was removed at operation. Incisional pain
is treated with small doses of narcotics.
Patients may go to the bathroom with
assistance the evening of operation if they feel so inclined. They may
ambulate on the first postoperative day, but should not bend or stoop
over for 4 to 5 days. Patients who have been on large doses of
carbamazepine preoperatively should be placed on decreasing doses
postoperatively to prevent withdrawal symptoms, usually manifested as
agitation.
The wound dressing is removed on the third
postoperative day, and the patient is instructed to shower and to
shampoo daily with mild shampoo. The patient is discharged from the
hospital on the fourth or fifth postoperative day, occasionally on the
third day. The sutures are removed on the seventh postoperative day.
 Postoperative Course, Results, and
Complications
Postoperative Course, Results, and
Complications
The patient may still have trigeminal
neuralgia on awakening from the anesthetic or may develop recurrence of
pain in the next few days. It should not be as severe as the
preoperative pain and should disappear promptly. If the pain is as
severe as it was preoperatively, or suddenly recurs with that severity,
it may be presumed that a causative blood vessel has been missed or that
the decompression was inadequate. Reoperation should be performed
early, as it is easier on the surgeon and the patient if done promptly.
Cerebrospinal fluid leaks are uncommon,
but if one occurs, a pressure dressing is applied to the wound, and
closed lumbar drainage is instituted using the method of McCallum and
colleagues.
Postoperative delayed headache, with or
without nuchal rigidity or fever, may occur from the fourth to seventh
postoperative day. The cerebrospinal fluid typically shows elevated
pressure and may show leukocytosis, but no bacteria appear on Gram stain
or culture. The fluid may be xanthochromic with an elevated protein
value and normal or low-normal sugar content. This syndrome of delayed
headache occurs in about 15 percent of patients. It must be
differentiated from infection. Lumbar puncture is often curative if the
opening CSF pressure is reduced halfway to 100 mmH2O, If symptoms
persist, a repeat lumbar puncture is performed and a single 10-mg dose
of dexamethasone given. In general one can expect that about one patient in 200 will have recurrent pain by
10 years after the operation.
Barba and Alksne in 1984 observed that
permanent relief is obtained in over 90 percent of patients who have not
undergone a prior destructive procedure, but in only 43 percent of those
who have had such a procedure. The implications of this important
observation are not yet clear. Are these patients just less amenable to
any therapy? Does the pre-existing, more peripheral lesion create a
pathophysiologic situation that makes the tic more difficult to treat?
Is the reverse situation also true (that is. for a failed microvascular
decompression followed by a destructive operation)? Should selective
sectioning of the portio major be performed in addition to the vascular
decompression in patients who have had recurrence after a prior
destructive procedure'). Results do not support the observation that
prior destructive procedures worsen outcome so severely.
The morbidity and mortality rates in
patients have decreased significantly owing to increased experience. to
the use of the lateral decubitus rather than the sitting position. to
auditory monitoring. In Janneta P.J series, more than 1700 microvascular
decompressions for trigeminal neuralgia were performed. with two operative deaths. the
first in 1971 and the second in 1976. The latter patient is actually
doubly reported in a series from another institution. They have had two
postoperative deaths after excision of tumors causing trigeminal
neuralgia, both in the 1970s. The major current morbidity consists of
the delayed headache syndrome. which is selflimited. Major cranial
nerve deficits consist of facial numbness in some patients in whom the
vascular decompression was particularly difficult and of hearing loss
in the ipsilateral ear.
The
incidence and prevalence of trigeminal neuralgia may increase as the
median age in our population rises. Microneurosurgeons who learn the
nuances of microvascular decompression will be able to perform
the operation safely and effectively. The data of others,
show that microvascular decompression offers the best quality of life,
best results, and least true morbidity of any major operative procedure,
for trigeminal neuralgia. To see one of the operations about MVD,
click here! |