|
Surgeons are quite adept at
restoring the continuity of a
disrupted peripheral nerve following
a peripheral nerve injury. Although
restoration of neural continuity may
be enough to treat some patients
with a peripheral nerve injury, many
patients will require additional
therapy. The surgeon must be
prepared to treat the patient's
dysfunction, not just the injured
nerve. Here a reviews the available
methods of restoring function to an
extremity following nerve injury.
A
variety of methods are available to
enhance the patient's functional
recovery. During the immediate
postinjury period, physical therapy
and splinting must be employed to
avoid skin and muscle contractures
and stiff joints. Contractures limit
the maximum range of motion achieved
by all other forms of therapy.
Muscle transfers and orthotics may
improve mobility in those patients
who do not achieve a full functional
recovery. The surgeon treating
peripheral nerve injuries must be
aware of the role that these
techniques can play when planning a
patient's therapy.
Here
reviews the functional deficits
incurred with upper extremity
peripheral nerve injuries, as well
as the potential for methods of
therapy designed to restore lost
function. A variety of treatment
schemes are presented. The merits
and disadvantages of each are
discussed.
 Physical Therapy
Physical Therapy
The need for maintaining joint
mobility cannot be overlooked by the
surgeon performing a peripheral
nerve repair. A reinnervated muscle
or tendon transfer cannot be
expected to move a joint beyond what
is allowed by the joint's intrinsic
limitations of motion. Passive range
of motion is preserved and joint
contractions avoided with the help
of a physical therapist. Most
patients will not need daily
supervised therapy once instructed
regarding the appropriate therapy.
The therapy often can be carried out
at home. The therapist can then
monitor the patient's progress at
regular intervals. Even the most
compliant patient, however, will
need to be monitored for early
contractures.
 Orthotics
Orthotics
Orthotics can be used to help solve
two fundamental problems encountered
in patients with an upper extremity
nerve injury. First, the orthotic
can be used to prevent or correct
soft tissue and joint contractures.
The preservation of a normal range
of motion will allow the patient to
take full advantage of later
reinnervation. Both static and
dynamic splints are available for
this purpose. The surgeon, working
with the orthotist, must choose a
splint that maximizes the patient's
use of the injured extremity but
that is not so cumbersome or
unsightly as to discourage the
patient's compliance. For instance,
a splint with a dynamic finger
extension assembly is ideal for a
secretary with a radial nerve palsy,
but it most likely would be too
cumbersome for a salesperson who is
self-conscious about appearance. It
is emphasized that fixed orthoses
must be used with caution since they
can contribute to contracture
formation.
Second, an orthotic device can be
used to bolster weakened movement.
This can be accomplished sometimes
simply by stabilizing a joint. For
instance, stabilizing the wrist of a
patient with radial nerve palsy in a
neutral or slightly extended
position will tighten the finger
flexion tendons, adding strength to
the patient's grip. The patient thus
may benefit from the transfer of
power from a forceful movement to a
weakened movement. In this case, the
orthosis improves grasp by enhancing
the normal tenodesis effect that
causes the finger to flex when the
wrist is extended. The patient with
a severe, permanent hand paralysis
may benefit from a mechanical device
that is triggered by one of the
patient's retained functions. These
devices accomplish movement with the
aid of electric motors or springs.
In addition, a number of prosthetic
devices are available to replace a
lost portion of an upper extremity.
 Neurotization
Neurotization
Despite reported success with regard
to the treatment of brachial plexus
lesions, the restoration of motor
function following a brachial plexus
avulsion remains a difficult
problem. Although nerve grafts
placed against the spinal cord could
theoretically coax anterior horn
cells to sprout viable axons, this
technique has not been used to treat
patients with brachial plexus
avulsions. Function can be restored
in this group of patients by either
muscle transpositions or
neurotization. Neurotization is a
procedure whereby intact axons are
rerouted to denervated neural
elements (Figure 1). Neurotization
is an established technique. The
spinal accessory and hypoglossal
nerves long have been used for
neurotization of the facial nerve.
In 1913 Tuttle reported his attempt
at neurotization of the injured
upper trunk of the brachial plexus
using intact elements of the
cervical plexus. The duration of
follow-up, however, was too short to
evaluate the results of his
procedure adequately. Although
Tuttle's case generally is
considered to be the first reported
case of neurotization, Cushing cited
a report of the utilization of the
radial nerve to innervate the median
nerve. The first successful
neurotization using intercostal
nerves was reported by Yeoman, and
the first successful neurotization
using either the elements of the
cervical plexus or intercostal nerve
was reported by Kotani et al.
Intercostal nerves and elements of
the cervical plexus remain the most
commonly used nerves for
neurotization in the upper
extremity.
The timing of the neurotization
procedure is critical. Two years
after the injury, muscle cells are
fragmented and the muscle atrophied,
At this time, restoration of
innervation will not result in
meaningful contraction, Since
innervation of the biceps brachialis
or supraspinatus will not be evident
until at least 7 months following
the procedure, neurotization should
be performed as soon as the
diagnosis of a complete avulsion is
established, If the patient is
evaluated at more than
1
year following the injury, or if the
recipient nerve is found to be
fibrotic at the time of inspection,
direct neurotization has little
chance for success. Patients who
have undergone a successful
neurotization procedure can retrain
the transposed nerves to serve a new
function, Cushing noticed that as
time passed, a patient with an
accessory to facial nerve
anastomosis could perform more and
more intricate facial movements
without concomitant shoulder
movement. Vera et al. further
documented this plasticity by serial
electromyograms performed on a
4-year-old boy who had undergone an
accessory to facial nerve
anastomosis, Animal experiments
performed to investigate the effects
of cross-neurotization indicate the
ability to reeducate the transposed
nerves to subserve their new job.
The Duke experience using
intercostal nerves to reinnervate
the biceps brachialis demonstrates
the plasticity of the nervous
system, Although initially the
biceps brachialis only contracts
with respiration, the patients soon
learn to contract their biceps
muscles independent of the
respiratory cycle, Eventually biceps
brachialis contraction is performed
without a conscious effort,
Available donor nerves contain a
relatively small number of axons as
compared to potential recipient
targets. Therefore, attempts at
neurotization of the trunks and
cords of the brachial plexus have
not been successful, as the
regenerating axons diffuse out along
disparate paths. It is best to
direct available donor axons into a
nerve just proximal to a single
recipient muscle. Most procedures,
therefore, are aimed at restoring
elbow flexion or shoulder abduction.
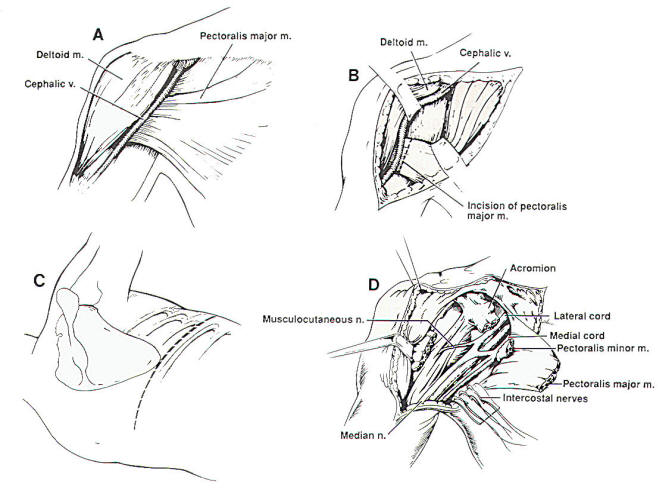
Fig-1
 Muscle and Tendon Transfers
Muscle and Tendon Transfers
Muscle transfers often are helpful
in restoring function to a paretic
upper extremity. A knowledge of this
technique aids the surgeon in
several ways, First, muscle
transfers may enhance function in a
patient with an irreparable nerve
injury. Second, the literature on
muscle transfers concentrates on the
functional mechanical deficit caused
by a nerve injury. It is important
for the surgeon to know and document
the functional evaluation of the
injured nerve. A knowledge of muscle
testing alone is inadequate. A
knowledge of the patient's
functional deficit is essential to
the physician who must initiate
physical therapy to avoid
contractures and plan future
operative procedures directed toward
returning the patient to a
productive life.
In
this section, the general principles
and the desired muscle transfers are
outlined, The technical details and
the merits of the variety of
available procedures are not
described, The interested physician
therefore is directed to the
literature detailing the technique
of muscle transfers.
The art of tendon and muscle
transfer has evolved over the last
130 years, A review of the rules
guiding successful tendon transfers
will lead to an understanding of the
limitations of these techniques,
Each time a tendon is removed for a
transfer, a joint is made less
stable or a movement weakened,
Therefore, each tendon transfer has
a price, The surgeon must decide
which transfers will be most
advantageous for the needs of the
individual patient. For instance, in
some patients, a powerful grasp is
important in carrying out work. In
these patients, tendon transfers to
strengthen finger flexion are
important. Other patients will do
perfectly well with a weakened
grasp. In those patients, such
transfers are superfluous.
In
planning a tendon transfer, the
surgeon must take into account
mechanical, tissue, and
rehabilitation considerations. The
mechanical considerations are
straightforward. The muscle
transferred must be of sufficient
strength and have sufficient
excursion to accomplish its new
task. All forearm muscles do not
have the same excursion. The laws of
mechanical physics, especially those
of vectors and levers, will
determine the movement accomplished
by the tendon transfer. For
instance, if a tendon crosses two
joints, its effect on the movement
at each joint is determined by the
direction of pull and length of the
lever arm affecting that joint.
Tissue factors must be taken into
account when planning a tendon
transfer and also in planning any
surgery that precedes the transfer.
Muscles and tendons must have an
adequate blood supply in their new
position. The tendon must be routed
through virgin tissue. A tendon
routed through scar tissue is likely
to develop adhesions that impede its
excursion. This principle must be
kept in mind when planning operative
procedures that precede the tendon
transfers. Prior to the transfer,
the target joint must have a full
passive range of motion. Maintaining
range of motion is accomplished by
preoperative physical therapy,
splinting, and occasionally the
surgical release of adhesions.
Finally, the most perfectly planned
tendon transfer procedure will not
be of benefit unless the brain can
be reoriented to use the muscle in
its new position. The patient must
have a sufficient mental capacity
and interest to participate in a
re-education program. This program
should be coordinated by a physical
therapist who has the time and
experience to help the patient use
the new transfer effectively. The
reeducation process is said to be
easiest when the donor muscle's
former role was synergistic with the
desired motor effect of the planned
transfer.
Most tendon transfers are carried
out only when one is certain that
there will be no further improvement
in the patient's paralysis. Some
authors advocate early transfers of
a few tendons to help avoid
contractures and to enhance function
while awaiting reinnervation. The
possibility of early transfers
should be considered in the patient
who is severely incapacitated by a
peripheral nerve injury.
 Sensory Retraining
Sensory Retraining
The importance of sensation in the
upper extremity often goes
unrecognized. In fact, loss of
sensation within the hand can be
quite debilitating. Even if the
patient recovers good motor
function, the ability of the hand to
carry out fine tasks will be limited
by poor sensory perception.
In
general, recovery of two-point
discrimination in the hand is poor
following the repair of an ulnar or
median nerve injury. The recovery of
sensation following peripheral nerve
repair can be improved greatly by
using sensory re-education
techniques. Dellon et al. first
reported near normal sensation in
four adults who had undergone a
median nerve repair followed by
sensory education. Several other
authors have corroborated these
results. In sensory re-education,
the patient stimulates the
hypoesthetic area with progressively
more intricate objects. At first,
the patient looks at the object
during stimulation to correlate the
new sensory signal with the visual
impression of the object. Then the
patient blindly picks up and tries
to identify the object. Using this
technique, two-point discrimination
is greatly improved. This
improvement may persist for years.
When a nerve injury leaves a portion
of the patient's palm or digit
anesthetic, some sensation can be
restored using neurocutaneous island
pedicle flaps. With this technique,
a flap of skin with a neurovascular
pedicle is moved from a less vital
area to a vital sensory surface. For
instance, the ulnar volar surface of
the ring finger can be transferred
along with its neurovascular pedicle
to the palmar surface of an
insensitive thumb. Some patients
will gradually reorient sensation in
the graft from the donor site to the
thumb. Unfortunately, the
transferred pedicle may develop a
cold sensation and hyperesthesia.
Rarely, sensory nerve transpositions
have been reported for restoration
of sensation into the hand.
In
the following section, specific
nerve injuries will be discussed.
The specific movements impaired and
intrinsic mechanisms of
compensation, or so called "trick
movements" are reviewed. The more
common methods used to restore lost
function are outlined.
 Axillary Nerve
Axillary Nerve
An
isolated axillary nerve injury
denervates the deltoid muscle and
thus greatly reduces the strength of
shoulder abduction. Most patients
still can raise the affected arm
over the head using external
rotation of the scapula as well as
the supraspinatus, the long head of
the biceps, and the clavicular
fibers of the pectoralis major
muscle. Unfortunately, the movement
lacks power.
Because the axillary nerve subserves
motor function primarily, repair of
the nerve with or without a nerve
graft offers an excellent chance of
restoring function. This, therefore,
is the preferred method of
treatment.
Several ingenious muscle transfers
using the trapezius, serratus
anterior, levator scapulae,
latissimus dorsi, and
sternocleidomastoid muscles have
been described to restore shoulder
function. These transfers seldom are
necessary for patients who have
suffered an isolated axillary nerve
injury but should be kept in mind
for treating patients who have
suffered concomitant paralysis of
other shoulder muscles. Patients who
have paralysis of most of their
shoulder musculature from a C5-6
avulsion are best treated with a
fusion of the scapula to the
humerus. The transfer of one or two
muscles will not benefit an unstable
shoulder. Neurotization of the
axillary nerve only has been
attempted rarely in order to restore
shoulder abduction. Neurotization
procedures of the supraspinatus
nerve using the distal branch of the
spinal accessory nerve or
intercostal nerves have been
described. Because of its small
sensory component, neurotization of
the axillary nerve should produce
results at least as good as similar
procedures for the musculocutaneous
nerve.
 Musculocutaneous Nerve
Musculocutaneous Nerve
The musculocutaneous nerve rarely is
injured in isolation. When such an
injury occurs, some elbow flexion
may be retained. The flexion in this
case is carried out by the pronator
teres and the brachioradialis
muscles. More commonly, however,
elbow flexion is lost as a part of a
more extensive brachial plexus
injury.
While awaiting return of elbow
flexion, passive exercises should be
initiated to maintain the full range
of motion of the elbow. Several
muscle transfers have been described
to reconstitute elbow flexion. The
original transfers described by
Steindler called for a transposition
of the origin of the finger
flexorpronator muscle group to a
position more proximal on the
humerus. Although this transfer
produces only weak elbow flexion,
still it is useful for treating
patients who have suffered an upper
brachial plexus avulsion. The
latissimus dorsi, pectoralis muscle,
and triceps all can be transposed to
strengthen elbow flexion.
Because elbow flexion is the most
vital function provided by the arm,
the musculocutaneous nerve most
frequently has been the target of
neurotization procedures. In the
Duke series, 16 patients had
neurotization of the biceps
brachialis by intercostal nerves and
4 additional patients had
neurotization of a free gracilis
muscle graft transposed into the
position of the biceps. Nine of
twenty patients (45%) obtained
useful antigravity strength and a
full range of elbow flexion
following surgery. Several other
authors have reported their
experiences using intercostal nerves
or elements of the cervical plexus
to reinnervate the biceps brachialis
(Table 1).
|
TABLE 1
- Shoulder Abduction |
|
Author
|
Number of
points |
Donor
Nerve |
Recipient
Nerve |
Results |
|
Kotani
(1973) |
1 |
Spinal
accessory |
Upper
trunk
|
1 good
|
|
Sedel
(1982) |
1 |
Accessory |
Posterior
cord |
1 poor
|
|
Brunelli
(1984) |
13 |
Cervical
plexus |
Suprascapula |
11 good,
2 fair
|
|
Narakas
(1985) |
2 |
Spinal
accessory |
Suprascapula
|
1 good, 1
poor |
|
8 |
Intercostal nerves |
Axillary |
4 good, 4
poor |
|
Solonen
(1984) |
9 |
Intercostal nerves |
Axillary
or suprascapular |
1 fair, 6
poor |
|
Yamada
(1991) |
3 |
Cervical
plexus |
Upper
trunk |
3 good
|
|
Samardzic
(1990) |
1 |
Spinal
accessory and
intercostals |
Axillary |
1 good |
 Radial Nerve
Radial Nerve
Because the radial nerve primarily
innervates muscle in the forearm and
contains relatively few sensory
fibers, a patient with a radial
nerve injury has a good prognosis
for recovery of some function
following primary anastomosis or
nerve grafting. A high radial nerve
injury will result in weakness of
the ulnar and radial wrist
extensors, extension of all five
digits of the metacarpophalangeal
joint, abduction of the thumb, and
extension of the thumb at the
interphalangeal joint. Weak
extension of the distal phalanx of
the thumb is preserved in many
patients by a slip of the abductor
pollicis brevis that attaches to the
extensor pollicis longus tendon. The
inability to stabilize the wrist and
the metacarpophalangeal joints
greatly weakens the patient's grasp.
This loss of wrist and finger
stability is responsible for the
greatest functional deficit. In some
patients, sensory fibers to the
dorsum of the hand travel along the
antebrachial cutaneous nerve. In
these patients, a radial nerve
injury does not cause any loss of
sensation. In any case, the sensory
loss incurred by a radial nerve
injury is of no functional
significance unless it is
accompanied by a painful neuroma.
Following radial nerve repair, the
patient should be instructed in
passive range of motion exercises to
prevent joint adhesions and
contractures of the web space
between the thumb and index finger.
Several types of orthoses have been
described to improve the patient's
function following a radial nerve
palsy. A simple volar cock-up splint
will hold the patient's wrist in
extension, increasing the strength
and accuracy of the patient's grip.
The patient must be instructed to
continue range of motion exercises
or the splint can lead to joint
contractions. Dynamic finger and
thumb assemblies will hold the
digits in extension while the
patient is at rest but will still
allow the patient to flex the
digits. The dynamic assemblies make
the splint more complicated and
cumbersome. Some authors have
advocated an early tendon transfer
to serve as an internal splint while
awaiting the return of motor
function. The most common procedure
uses an end-to-side anastomosis of
the pronator teres and to the
extensor carpi radialis brevis. This
transfer will stabilize the
patient's wrist and will not
interfere with the normal
musculature once nerve regeneration
occurs. Muscle transfers are very
successful at reducing the
functional deficit that occurs
following a radial nerve palsy.
Because all of the ulnar and median
innervated extrinsic muscles of the
hand are available for transfer, a
large number of different transfers
have been described. Wrist extension
is most commonly restored by a
transfer of the pronator teres to
the radial wrist extensor tendons.
Finger extension is achieved by a
transfer of the flexor carpi
radialis or a single tendon of the
flexor digitorum sublimis to the
extensor digitorum common. Thumb
extension and abduction can be
achieved by transferring the
palmaris longus, flexor carpi
radialis, or one tendon of the
flexor digitorum sublimis to the
extensor pollicis longus tendon.
 Ulnar Nerve
Ulnar Nerve
The repair of a disrupted ulnar
nerve proximal to the elbow is
unlikely to result in a good return
of lost motor function. Such repair
still should be performed in an
attempt to restore sensation to the
ulnar side of the hand. A complete
proximal ulnar nerve lesion will
result in weakness of the flexor
carpi ulnaris, flexor digitorum
profundus to the small and ring
finger, adduction pollicis, and
several intrinsic muscles in the
fingers. Sensation will be lost over
the ulnar one-and-a-half digits and
adjacent hand.
Weakness of the flexor carpi ulnaris
and ulnar portion of the flexor
digitorum profundus muscles does not
pose a significant problem to most
patients. Wrist flexion is still
carried out by the flexor carpi
radialis and palmaris longus
muscles, although there is some loss
of strength and radial deviation of
the wrist. Strong lateral pinch of
the thumb is weakened by the loss of
the adductor pollicis and the deep
head of the flexor pollicis brevis
muscles. Some weakened thumb
adduction can be carried out by the
abductor pollicis brevis muscle if
the thumb is held in front of the
palm. If the thumb is held in the
plane of the palm, weak adduction is
achieved by combined actions of the
extensor pollicis longus and the
flexor pollicis longus muscles The
telltale flexion of the distal
phalanx of the thumb with lateral
pinch performed by the extensor
pollicis longus results in Froment's
sign. This weakened adduction
diminishes the thumb's effectiveness
as a stabilizer during a power grip.
Interossei weakness causes loss of
finger abduction and adduction. This
manifests as an instability of the
index finger during fine pinch. Some
finger abduction is performed by the
finger extensors when the fingers
are allowed to flex forward away
from the palm. Loss of the
interossei muscles also weakens
flexion of the metacarpophalangeal
joint and results in a weakened
"power grip" (Figure 2).
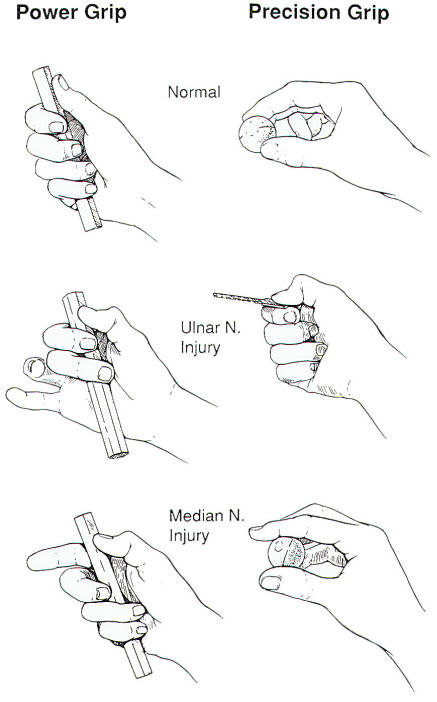
Fig-2
This loss of coordinated
metacarpophalangeal flexion
decreases the patient's ability to
wrap his or her fingers around an
object. With the additional loss of
the lumbricale muscles to the ring
and small fingers, hyperextension of
the metacarpophalangeal joints
results. This hyperextension in turn
decreases the patient's ability to
straighten the interphalangeal
joints, leading to clawing of the
ulnar two fingers. Loss of the
intrinsic muscles to the small
finger results in flattening of the
hand, which further weakens grip. A
chronically abducted small finger
will inhibit the patient's ability
to quickly put the hand into a
contained space such as a pocket or
shirt sleeve. In summary, the motor
deficits that occur with an ulnar
nerve injury greatly weaken the
patient's "power grip," i.e., the
prehensile position in which the
object is clamped by the flexed
fingers and stabilized by the thumb.
Precision grip is much less impaired
(Figure 2).
Exercise and splinting may be
necessary to avoid flexion
contractions of the ring and little
fingers' interphalangeal joints. A
splint with a metacarpophalangeal
extension stop assembly will prevent
metacarpophalangeal joint
hyperextension and finger clawing.
Some authors have advocated internal
splints to coordinate the residual
motor function of the hand while
nerve regeneration is taking place.
To this end, a single tendon from
the flexor digitorum sublimis is
used to stabilize the
metacarpophalangeal joints of the
ring and little fingers and to
improve adduction of the thumb.
It
is not possible to transfer enough
tendons to restore each muscle lost
following the loss of the ulnar
nerve, so the surgeon must
concentrate on restoring functions
that are important to each
individual patient. Weakness of the
flexor digitorum profundus and
flexor carpi ulnaris is only of
consequence if the patient's
occupation requires a strong,
accurate grasp across the entire
palm. Grasp can be improved by
attaching the flexor digitorum
profundus tendons of the ring and
small fingers to that of the middle
finger or by attaching the extensor
carpi radialis tendon to the two
weakened tendons.
A
number of transfers have been
devised to improve flexion of
metacarpophalangeal joints. If the
metacarpophalangeal joints can be
kept from hyperextending, the
extensor digitorum communis will
adequately extend the
interphalangeal joints. Most often,
the extensor carpi radialis longus
or flexor carpi radialis tendon is
used to flex the ulnar
metacarpophalangeal joints.
Thumb adduction is strengthened most
often using the brachioradialis
muscles, a single tendon from the
flexor digitorum sublimis, or less
commonly, the extensor digit
proprius. A pulley is established
with a fascial sling, or by passage
of a tendon around the metacarpal of
the long finger so that the thumb is
pulled horizontally in the plane of
the palm.
The strengthened thumb adductor will
still pinch against the weakened
index finger abductor. If the
patient's occupation requires a
strong lateral pinch, index finger
abduction can be strengthened by a
transfer from the extensor pollicis
brevis and fusion of the
metacarpophalangeal joint of the
thumb. Cupping of the metacarpal
arch and little finger adduction are
accomplished by a transfer from the
extensor digiti minimi or less
commonly from the flexor digitorum
superficialis.
These transfers are designed to
increase the strength and accuracy
of the patient's grip and pinch.
Some sensibility can be
re-established by using a
neurovascular cutaneous island
graft.
 Median Nerve
Median Nerve
A
high median nerve Injury is a
disabling condition. Denervation of
the long flexors to the distal
interphalangeal joint of the thumb,
index, and long fingers, and the
primary flexors of the proximal
interphalangeal joint of all four
fingers weakens the patient's power
grip. Some paralysis is partially
compensated for by intact muscles.
The distal phalanx of the long
finger usually flexes synchronously
with the distal phalanx of the ring
finger, as the two tendons of the
flexor digitorum longus muscle share
a common, ulnar innervated muscle
belly. Although loss of the flexor
digitorum sublimis decreases the
strength of the grasp and
independent finger flexion, the
intact flexor digitorum profundus to
the ulnar digits flexes the proximal
interphalangeal joint along with the
distal interphalangeal joint.
Precision pinch is impaired by loss
of thenar palmar abduction
(abduction perpendicular to the
palm) and opposition (internal
rotation) movements initiated by the
opponens pollicis, abductor
pollicis, and to a lesser extent,
flexor pollicis brevis (Figure 2))·
The flexor pollicis brevis and
longus muscles add strength to the
pinch. Some palmar abduction and
internal rotation of the thumb occur
in approximately one-third of
patients with a medial nerve injury
as a result of a dominant ulnar
innervation of the flexor pollicis
brevis muscle.
An
injury to the median nerve proximal
to the elbow weakens wrist pronation
and flexion. Wrist flexion is still
carried out in the absence of the
flexor carpi radialis and digitorum
flexors by the intact flexor carpi
ulnaris and abductor pollicis longus
muscles. This compensated motion
usually occurs with concomitant
ulnar wrist deviation.
It
must be remembered that median nerve
injury robs the patient of critical
sensory perception in the tips of
the thumb and index finger. Without
this sensation, fine pinch is of
little value. While awaiting
reinnervation of the thenar eminence
following a median nerve repair, the
surgeon must guard against
contracture of the dorsal skin of
the thenar web. This common
contracture limits opposition of the
thumb and, if it occurs, should be
treated by splinting or even
surgical release. Mobility of the
interphalangeal joints of the thumb
and index fingers also must be
maintained. Some surgeons advocate
early muscle transfers to act as
internal splints.
Several orthotic devices have been
developed to oppose the thumb and
index fingers, stabilize the thumb,
and prevent web space contractures.
Accessories may be added to prevent
wrist dorsiflexion. Following an
irreparable injury to the hand,
spring- or electrically driven
devices can oppose the thumb and
index fingers providing the patient
has retained a useful pinch
mechanism.
Several muscle transfers have been
described to restore opposition of
the thumb following an injury of the
median nerve. The flexor digitorum
sublimis muscle is the most commonly
used motor for an opponensplasty
following a low median nerve injury.
Following a high median nerve injury
that has paralyzed the long finger
and wrist flexors, thumb opposition
can be partially restored by a
tendon graft attached to a
transposed extensor muscle such as
the extensor carpi ulnaris, extensor
indicis proprius, or extensor
digiti minimus.
Because the ulnar portion of the
flexor digitorum longus is
innervated by the ulnar nerve,
simultaneous flexion of all of the
fingers can be provided by
tenodising (suturing together) the
long flexor tendons of the small and
ring fingers to those of the long
and index fingers. This only will
allow the fingers to flex in mass
and may result in a "swan neck"
deformity of some or all of the
digits. Independent flexion of the
index finger is important to the
patient who depends on a precision
pinch. This can be accomplished by a
transfer of the extensor carpi
radialis longus tendon to the flexor
digitorum longus tendon of the index
finger. Flexion of the thumb is
accomplished by attaching the
brachioradialis muscle to the tendon
of the flexor pollicis longus. The
ulnar deviation frequently observed
in wrist flexion carried out solely
by the flexor carpi ulnaris can be
corrected by splitting that muscle's
tendon and attaching one slip of the
split tendon to the insertion of the
flexor carpi radialis insertion.
Sensation can be partially restored
to the ulnar volar surface of the
thumb and opposing surface of the
index finger by rotating a double
cutaneous island with neurovascular
pedicle from the opposing surfaces
of the ring and small fingers.
|