|
Confusing
anatomy is often encountered during operations on complex
dysraphic lesions in the lumbosacral canal. It is common to see
nerve roots embedded in lipoma or scar tissue, or they may not
be easily distinguishable from fibrous adhesion bands. Sometimes
nerve roots that are bundled tightly by abnormally thickened
arachnoid can look like a thickened filum terminale. Also, the
transition between a functional but structurally deformed conus
and an intramedullary lipoma is not always visually apparent.
Thus, some objective means to identify the sacral nerve roots
and the conus is necessary to ensure preservation of these
neuronal structures. In addition, in some cases of complex
transitional lipomas, the tip of the conus is tautly suspended
by low sacral roots that are short, stout, and fibrotic. An
assessment of their functional integrity is useful for
determining whether dividing them, in order to complete the
untethering process, would lead to unacceptable loss of
sphincter function.
The first
sacral and lower lumbar roots are recognized readily by
intraoperative nerve stimulation while palpating for
contractions of the respective segmental muscle groups through
the surgical drapes. Identification of the lower sacral roots
and functional quantification of these roots and their
corresponding medullary connections, however, require some
objective assessment of perineal sensation and sphincter
function.
 Modality for Sensory Monitoring of S2-4 Segments
Modality for Sensory Monitoring of S2-4 Segments
The
assessment of evoked responses generated by directly stimulating
parts of the sex organs, urethra, and anal canal constitutes the
mainstay of sensory monitoring of the lower sacral segments.
Monitoring of such responses is most useful when the distal
conus or dorsal nerve roots are being rather strenuously
handled, as in certain difficult resections of large
transitional lipomas or during removal of the median fibrous
sleeve of a Type I split cord malformation. The latency and
amplitudes of the waveforms are exquisitely sensitive to
structural deformation and ischemic changes to the central
sensory pathways. Sensory evoked response monitoring is less
useful in the identification of sacral sensory roots, because
the responses are generated by end organ stimulation. Cortical
responses generated by direct dorsal root stimulation give much
less predictable waveforms, which are not stable enough for
foolproof identification purposes.
 Anatomy
Anatomy
The
peripheral nerves that supply the bladder, anal canal, and
perineal skin, all potentially available for stimulation, are
divided into three main groups.
1.The
pudendal nerve is the primary somatic nerve to this region. The
pudendal motor neurons innervating the external sphincter and
pelvic floor originate from Onuf's nucleus in the anterior horn
of the S2 to S4 cord segments. The sensory fibers come from the
corresponding dorsal root ganglia. The mixed fibers course via
the S2, S3, and S4 roots to exit the spinal canal through the
sacral foramina (Figure-1). Somatosensory impulses travel in
this nerve from receptors located in the skin of the genitalia
and perineum, the pelvic floor, and bulbocavernosus muscles, as
well as in the mucosa of the distal urethra and anus. Motor
fibers in the pudendal nerve innervate the bulbocavernosus
muscle, external urethral sphincter, external anal sphincter,
and pelvic floor muscles. The pudendal nerve is the most easily
accessible nerve for evoked response testing.
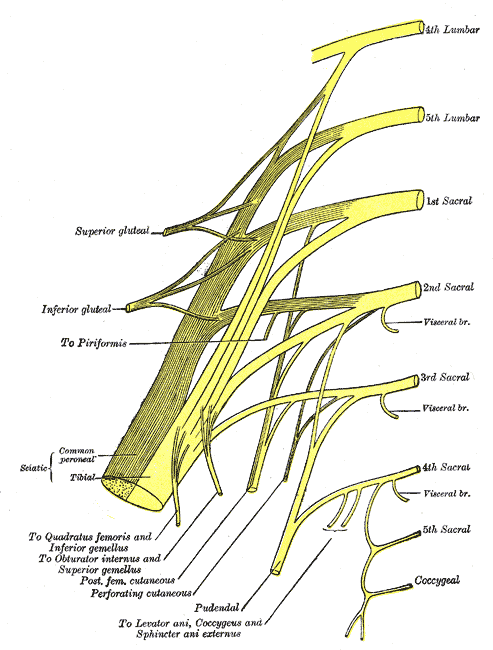
Fig-1:
Schematic representation of the pudendal nerve and branching.
3.The
pelvic splanchnic nerves supply the sacral parasympathetic
innervation to the pelvic organs. The motor neurons in this
nerve originate in the S2 to S4 cord segments, slightly more
caudal than the pudendal motor neurons. The fibers are
distributed to the pelvic organs via the S2 to S4 nerve roots
and inferior epigastric plexus. The pelvic nerve carries sensory
afferents from the proximal urethra, bladder wall, prostate,
seminal vesicles, and rectum. Motor innervation is primarily to
the detrusor muscles, the corpus cavernosus, the rectum, and
probably the upper smooth-muscle portion of the external
urethral sphincter. Evoked responses can be elicited on
stimulation of the proximal urethra and bladder, presumably due
to activation of the pelvic sensory fibers.
3.The
hypogastric nerve plexuses carry autonomic (sympathetic) fibers
from the intermediolateral cell column of the T11-L2 spinal cord
segments. The preganglionic fibers course via the paravertebral
sympathetic chain ganglia, inferior mesenteric plexus, superior
hypogastric plexus, and finally the inferior hypogastric plexus.
The postganglionic fibers are distributed to the smooth muscles
of the bladder neck, the smooth-muscled internal urethral
sphincter, the parasympathetic intramural ganglia of the
detrusor muscles and probably the intrinsic portion of the
external urethral sphincter. The postganglionic fibers also
share connections with plexuses around the rectum and anal
canal, seminal vesicles, ductus deferens, prostate, and corpus
cavernosus in the male, and vagina in the female. It is
uncertain how much the afferent component of the hypogastric
nerves contributes to the evoked response in humans.
 Cortical Sensory Evoked Response
Cortical Sensory Evoked Response
Standard
recording of the cortical evoked response is made by 5-mm silver
or gold-plated cup electrodes or dermal needle electrodes
sutured to the scalp. The electrode impedance should be kept
below 2000 Ω. The active
recording electrode is placed in the midline, approximately 2 cm
behind the Cz electroencephalographic recording site according
to the International 10-20 Electrode Placement System. This has
been demonstrated to give maximum cortical response on
stimulation of the penile and clitoral skin. The reference
electrode can be placed at a number of sites, although the
forehead (Fpz) is convenient and gives a good waveform. Stimuli
are delivered at a rate of 3.5 to 5.0 per second, with
approximately 2.5 to 3.0 times the threshold intensity. The
recording console consists of high- and low-frequency filters to
keep the band pass at 30 to 1000 Hz. The sensitivity of the
signal amplifier is usually set at 2 to 10
µV per division. About 250
to 350 responses are averaged to ensure reproducibility of the
reading, but weak and unstable signals from severely damaged
conuses may require up to 1000 responses to generate an
interpretable waveform.
 Pudendal Dermatomal Evoked Response
Pudendal Dermatomal Evoked Response
The most
commonly used form of pudendal nerve evoked response utilizes
stimuli applied to the sensory domain of the dorsal genital
nerve. In the male, the dorsal nerve of the penis can be
stimulated either bilaterally or unilaterally using 5-mm cup
electrodes placed 2 to 3 cm apart at the base of the penis, with
the cathode proximal to the anode. Stimuli up to 3.0 or 3.5
times threshold are well-tolerated. In the female, the dorsal
nerve of the clitoris is stimulated by 5-mm cup electrodes or
fine dermal needle electrodes fixed bilaterally to the cleft
between the labia major and labia minor. The anodes are placed
adjacent to the clitoris bilaterally and the cathode
approximately 2 cm posterior to the anode.
The
averaged cortical pudendal evoked response has a similar
morphology as the responses obtained from stimulation of the
posterior tibial or peroneal nerve. The response has a fairly
characteristic "M" pattern, with an initial positive deflection
followed by a constant negative, positive, negative, positive
waveform. Injury to the S2-4 roots or cord segments is
manifested by lengthening of the P1 latency and
decreased amplitude of the triphasic waves (Figure 2).
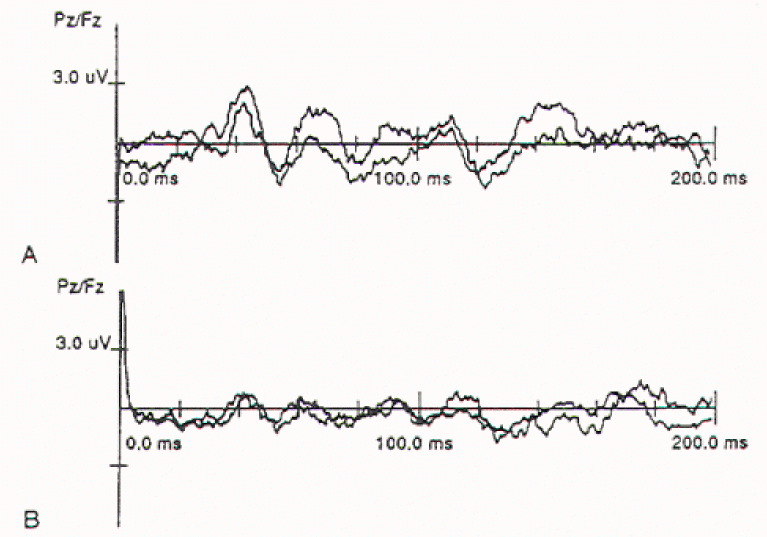
Figure 2.
Cortical pudendal nerve evoked responses obtained from a male
child with a Type I split cord malformation. The neurological
deficits are much worse in the left leg. All responses are
recorded from Pz referenced to Fz.
A)
Tracing obtained by stimulating the right dorsal
nerve of the penis. B) Tracing obtained by stimulating the left
dorsal penile nerve. Note the significant reduction in amplitude
in the left responses.
 Urethral Evoked Response
Urethral Evoked Response
Cortical
evoked responses of very similar morphology and latencies can be
obtained using stimulating electrodes embedded in a catheter
inserted into the bladder. The catheter has a balloon at its
tip, which can be pulled back snugly for anchorage. The location
of the urethral electrodes can be kept reasonably constant to
eliminate movement artifacts and interference.
 Anal
Evoked Response
Anal
Evoked Response
Electrode-bearing catheters can also be inserted into the anal
canal for measurement of anal evoked responses. The catheter is
anchored by double balloons, the inner one within the anorectal
junction and the outer one wedged at the anal verge. The
cortical anal responses do not differ from the urethral
responses or the pudendal dermatomal responses.
 Spinal
Evoked Response
Spinal
Evoked Response
Evoked
responses can be recorded by electrodes placed on the skin over
the spine in humans. They reflect the afferent volley traversing
the dorsal columns. The responses progressively increase in
latency at more rostral recording locations. Spinal evoked
responses are relatively easy to obtain in children, but the
amplitudes and waveform definition decrease with age, such that
by mid-teenage years, these responses are more difficult to
obtain, as in the case of adults. The response over the mid-tolower
lumbar spine consists of an initially positive triphasic
potential, representing the volley as it ascends the cauda
equina. Over the caudal thoracic spine, the response consists of
an initially positive, predominantly negative triphasic wave,
the negative component of which has several peaks or
inflections. The initial portion of this response arises in the
intramedullary continuation of the dorsal root fibers, and the
subsequent portion reflects synaptic activity concerned with
local reflex mechanism rather than the propagation of the
response to more rostral cord levels. From the mid-thoracic to
the cervical levels, the response consists of small, triphasic
potentials that are difficult to follow, presumably arising from
multiple ascending pathways including the dorsal and
dorsolateral columns.
The only
consistent spinal pudendal response has been from stimulation of
the dorsal nerve of the penis. The recording electrodes are
usually fixed at the T12-L1 interspinous space. The response has
a morphology comparable to the spinal response from the
posterior tibial and peroneal nerves but with smaller amplitudes
and a much shorter latency (Figure 3). The spinal pudendal
evoked response is sometimes not measurable in overweight
individuals, but its presence yields useful information
concerning peripheral sensory conduction from the penis since it
bypasses the central conduction pathway rostral to the thoracic
levels. Because the cortical pudendal evoked response has
similar latency with the cortical posterior tibial response, the
central conduction time involved in the pudendal pathways must
be considerably longer than that in the posterior tibial
pathways.
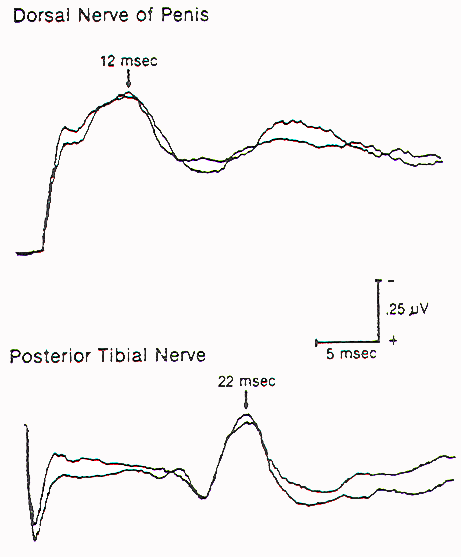
Figure 3.
Spinal pudendal evoked responses recorded over the T12-L1
interspinous space on stimulation of the dorsal nerve of the
penis (upper), and spinal responses on stimulation of the
posterior tibial nerve (lower). Note the much shorter latency of
the pudendal response.
 Modality for Motor
Monitoring of the S2-4 Nerve Roots
Modality for Motor
Monitoring of the S2-4 Nerve Roots
Pudendal sensory evoked responses are useful in
monitoring intraoperative injury to the conus and lower
sacral sensory nerve roots, but they are neither
qualitatively nor quantitatively suitable for the
identification of the lower sacral roots (especially the
motor roots) or conus from non-neural elements.
Intraoperative identification requires some way of
measuring the oneto-one stimulus-to-response coupling
of end organ function when the nerve root in question is
being stimulated. For the lower sacral roots, this means
the assessment of sphincter function.
 External Anal Sphincter
Electromyography
External Anal Sphincter
Electromyography
External anal sphincter electromyography (EMG) has long
been found useful as a qualitative tool for studying
anorectal closure function and disorders. The EMG
electrodes are either embedded in an anal plug or anal
balloon, which is placed into the anal canal, or are in
the form of needles inserted directly into the external
anal sphincter transmucosally. The needle electrodes are
more reliable because they are not subject to
dislodgement or to having mechanical artifacts during
contraction of the sphincter itself; however, accurate
and secure placement of the needles requires some
expertise and is initially best done by the
neuro-urologist. The grounding plate is pasted on the
patient's thigh, and EMG recordings are made using a
standard bladder diagnostic unit. The sensitivity of the
recording stylus is adjusted so that minimal deflection
occurs at rest. With stimulation of the lower sacral
motor roots, the stylus gives a discrete one-to-one
spike-deflection, much different than the baseline.
 External Anal Sphincter
Pressure Monitor
External Anal Sphincter
Pressure Monitor
The
external anal sphincter EMG requires bulky and expensive
equipment, as well as the availability of someone expert
in the accurate placement of the needle electrodes. An
alternate method of monitoring anal sphincter function
is the direct measurement of the "squeeze pressure"
induced by sacral root stimulation using a
pressure-sensitive balloon inserted in the anal canal.
This technique is simple and noninvasive, requires no
special expertise, utilizes inexpensive, portable
equipment, and produces easily interpretable pressure
waves which are semi-quantitative and virtually
unaffected by other electronic components in the
operating room that are known to cause annoying baseline
noise in an EMG recording.
 Physiology
Physiology
The
relationship between EMG and contractile strength in a
longitudinal muscle was first defined by Lippold, who
found a linear relationship between the integrated
action potentials on the EMG and the tension generated
by voluntary isometric contractions of the human
gastrocnemius. This linearity was explained by the fact
that an increase in contractile strength of a muscle is
brought about either by a spatially random increase in
the number of contracting motor units or by random
increments of discharge frequencies of the active units;
in both situations, the integrated electrical output of
the muscle would increase proportionately. The same
linear relationship was also demonstrated in the
external anal sphincter by Schweiger, who made
simultaneous recordings of sphincter EMG and anal canal
pressures with an anal balloon. These data support the
validity of using squeeze pressure, instead of sphincter
EMG, to monitor the functional status of the lower
sacral motor neurons.
In
order for the anal pressure monitor to be operational,
some sphincter function must be present. Theoretically,
a severely damaged motor nerve with only enough viable
axons to generate a barely visible EMG would produce no
measurable squeeze pressure; in such a situation, the
EMG might be more sensitive. However, such a nerve would
not provide useful sphincter function for the patient,
and its preservation is of doubtful value. In the
author's experience, any external anal sphincter that
could generate enough voluntary or reflex (as in the
bulbocavernosus or anal wink reflex in the infant)
contractions to be appreciable by preoperative digital
examination should produce recognizable pressure spikes
on the anal pressure monitor. This anal balloon monitor
is therefore sufficiently sensitive for the practical
purpose of sacral root and conus identification.
 Anatomy
Anatomy
The
external anal sphincter consists of a bulky deep part, a
fusiform superficial part, and a subcutaneous part
decussating behind and in front of the anus. It encloses
the lower part of the levator ani, the anorectal
junction, and the anal canal in the shape of a funnel.
The internal anal sphincter arises from the muscular
coats of the rectum and insinuates itself between the
rectal mucosa and the upper portion of the funnel.
The
external anal sphincter is innervated by the pudendal
nerve. This arises from the anterior division of S2 and
S3 and both divisions of S4, enters the pudendal
(Alcock's) canal through the lesser sciatic foramen, and
divides into two main branches just proximal to the
urogenital diaphragm. The proximal branch, the inferior
hemorrhoidal nerve, supplies the striated muscles of the
external anal sphincter; the distal branch, the perineal
nerve, supplies the external urethral sphincter. The
internal anal sphincter, composed of smooth muscles, is
innervated by the hypogastric nerve, derived from the
intermediolateral (sympathetic) columns of L1 and L2.
Stimulation of the S2, S3, and S4 roots, therefore,
activates only the external and not the internal anal
sphincter. Furthermore, unless there is localized
disease or trauma to the pudendal branches at the
urogenital diaphragm, activity of the external anal
sphincter reflects function of the external urethral
sphincter.
The
anal pressure balloon described here is an elongated
ellipsoid selected specifically to pick up activities
from all three parts of the external sphincter funnel.
Its elongated span also minimizes the possibility of
accidental dislodgement by contractions of the pelvic
musculature induced intraoperatively. Although the
elongated balloon will also pick up contractions of the
internal anal sphincter, the latter is never activated
by the nerve stimulator or by manipulation of the lower
sacral spinal cord or nerve roots because its nerve
supply is from L1 and L2. However, being made up of
smooth muscles, the internal sphincter does have
spontaneous rhythmic contractions that will be
registered by the balloon, and these must be
distinguished from the stimuli-generated pressure spikes
from the external anal sphincter.
 Equipment
Equipment
The
pressure sensor is a double-lumen balloon catheter
ordinarily used for intraluminal angioplasty (Figure 4).
The ellipsoidal balloon is made of treated polyethylene,
which does not stretch or deform at high inflation
pressures, so that a high degree of sensitivity to
circumferential squeezing can be maintained. The central
infusion catheter concentric with the balloon is not
actually being used in the pressure measurement but
functions effectively as a stent for easy balloon
insertion. The balloon comes in different sizes, but the
3 x 0.8-cm (inflated diameter) balloon should fit almost
any patient, from infants to large adults.
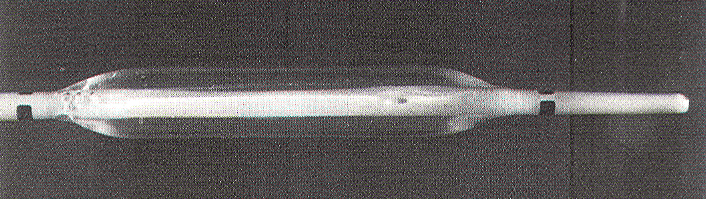
Figure 4. Double-lumen polyethylene balloon catheter.
The infusion lumen is not involved in the pressure
measurement but merely serves as a stent.
The
balloon is held vertically with the tip down, and is
maximally deflated and inflated several times with water
to expel all air bubbles. It is then connected to a
Bentley Model D240 pressure transducer, which displays
the pressure tracing on a two-channel Datascope Model
870 monitor (Figure 5). Although the baseline pressure
of the balloon, which can be adjusted by varying the
amount of water used, does not affect the actual
pressure measurement, it should be kept within a range
that allows the monitor to give good-sized pressure
waves in the usual sensitivity setting. The optimal
condition is when the balloon is rendered just turgid
(with 0.8 ml water for the 3 x 0.8-cm balloon) and when
the sensitivity on the Datascope monitor is set at 25 (1
cm on the screen is calibrated to 25 torr). The balloon
is inserted into the anal canal until its posterior end
is just visible at the mucocutaneous junction and then
taped securely to the gluteal skin.
One
cutaneous electrocardiography (ECG) electrode is pasted
over each iliac crest and a third on the right upper
thigh. The ECG tracing is displayed continuously on the
second channel of the Datascope screen (Figure 6)

Figure-5. Simple
assembly, consisting of the balloon catheter, the
Bentley pressure transducer, the injecting syringe and
stop-cocks and the 2-channel Datascope monitor.
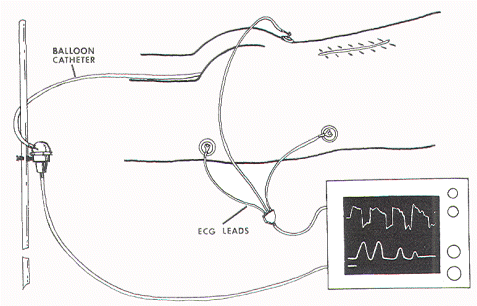
Figure 6. ECG electrodes are placed over the iliac
crests and the thigh to register the stimulus artifact.
 Technical Points
Technical Points
Intraoperative nerve stimulation is done with a
disposable monopolar nerve locator-stimulator using 3 V
and three variable current intensities: 0.5, 1, and 2
mA. The monopolar stimulator is chosen over the bipolar
stimulator because only the former will generate
sufficient volume-conducted current to produce an
obvious stimulus artifact on the ECG when any tissue is
touched by the monopolar electrode. When a lower sacral
root is stimulated, the combined ECG stimulus artifact
and the pressure spike from the external anal sphincter
form an easily recognizable electromechanical couple on
the monitor (Figure 7).
There are two advantages in having this
electromechanical couple. 1) The stimulus artifact
eliminates the possibility of a faulty stimulator or
faulty stimulation technique because even at 0.5-mA
current, the monopolar electrode always produces a
prominent spike on the ECG tracing. If an artifact is
present without a corresponding pressure spike at high
current intensity, the tissue stimulated does not
innervate the external anal sphincter; if neither
stimulus artifact nor pressure wave is obtainable, then
the nerve stimulator is faulty (Figure 8).
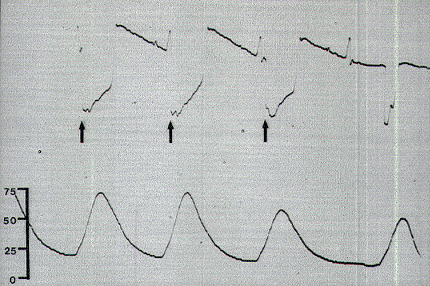
Figure 7. Monopolar stimulations of the S3 root in a
5-year-old child. Note the prominent stimulus artifact
on the ECG tracing (arrows) coupled with large pressure
spike waves measured at 50-torr peak values. Scale in
torr.
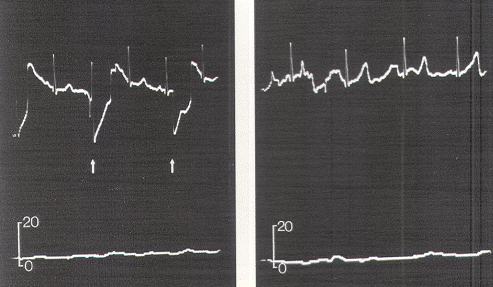
Figure 8. Use of the EGG. A) Stimulation of a
nonfunctional element (fibrous adhesion band) showing
only EGG stimulus artifacts (arrows) but no pressure
response. Scale in torr. B) Recognition of a faulty
nerve stimulator when neither an EGG stimulus artifact
nor a pressure response is detectable. Scale in torr.
2)
Involuntary, rhythmic activity of the internal anal
sphincter has been noted as spontaneous 5-to10-torr
waves with a frequency of 10 to 30 per minute, which may
be confused with external anal sphincter activities
except for the fact that these spontaneous waves are
completely out of phase with the ECG stimulus artifacts
(Figure 9).
During nerve stimulation, the cerebrospinal fluid must
be continuously suctioned away from the stimulation site
to prevent current dispersion. With this precaution,
supramaximal stimulation of the small sacral roots of
infants and young children can usually be accomplished
with 0.5 mA. The larger roots of adults sometimes
require higher amperage, as does direct conus
stimulation. Unilateral S2, S3, or S4 stimulation
consistently generates a peak pressure of 40 to 70 torr,
in line with recordings reported by Lane of 60 to 125
torr pressures with voluntary (bilateral) contraction in
normal adults. Even in young infants, peak pressure
responses are generally above 40 torr. S3 stimulation
produces the strongest and most consistent response in
the external anal sphincter. Direct stimulation of the
conus also results in waves of comparable peak values
but usually with a wider base, probably because of
multilevel and bilateral recruitment of anterior horn
cell units (Figure 10).
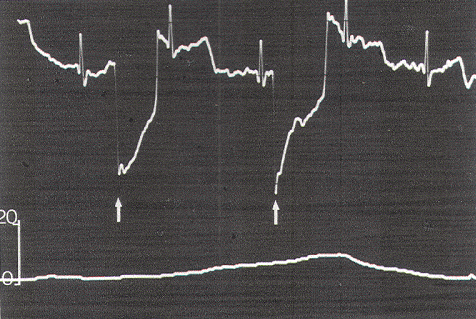
Figure 9. Use of the EGG. Spontaneous lower pressure
waves «10 torr) from the internal anal sphincter are
completely out of phase with the EGG stimulus artifacts
(arrows). Scale in torr.
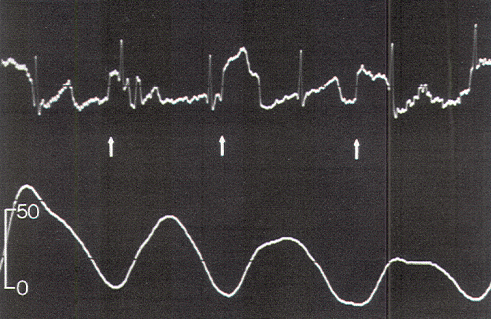
Figure 10. Direct stimulation of the conus in a
3-year-old boy, generating pressure waves with a wide
base and irregular blunted peaks. Stimulus artifacts are
indicated by the arrows. Scale in torr.
Occasionally, a small pressure wave of less than 7 torr
follows S1 stimulation (which does not innervate the
sphincters) because of compression on the protruding
proximal portion of the balloon by the medial inferior
fibers of the gluteus maximus. Although such "ripple
waves" are easily differentiated from the tall spike
waves of healthy lower sacral roots, they could be
mistakenly construed as the subdued responses seen with
partially damaged S2, S3, and S4 roots. This confusion
is eliminated if care is taken to secure the posterior
end of the balloon just above the mucocutaneous
junction.
Stimulation of the filum terminale and nonneural
tissues always produces a stimulus artifact but not a
pressure wave. Thus, the S2, S3, and S4 roots and the
conus can be distinguished from the S1 and lumbar roots,
the filum, lipoma, fibrous adhesions, and other
nonfunctional fibroneural bands, such as an occult
myelomeningocele. The ECG artifact and pressure wave
relationships are summarized in Table 1.
|
Table 1.
Interpretation of Stimulus (ECG) Artifact and
Pressure Response Relationship |
|
Stimulus (ECG)
Artifact |
Pressure
Response |
Interpretation |
| - |
- |
Faulty
stimulator |
| + |
-; spike waves
40-75 torr |
S2,S3,S4,
conus medullaris |
| + |
- or ripple
waves (<7 torr) (and plantar flexion) |
S1 |
| + |
- |
Lumbar roots,
filum, non-neural tissues |
|
No stimulation |
Rhythmic waves
10-30/min < 10 torr |
Internal anal
sphincter spontaneous activity |
 Clinical Use of the Anal Sphincter Function Monitors
Clinical Use of the Anal Sphincter Function Monitors
The anal
sphincter function monitors (EMG or pressure balloon) have been
found useful in the following circumstances.
1.
Functional sacral nerve roots embedded in large lipomas may be
detected and traced through a sometimes aberrant course to their
exit foramina. This is particularly useful in transitional
lipomas that involve the dorsal as well as the ventral portions
of the conus
2. Sacral
nerve roots can be distinguished from fibrous adhesion bands.
3. Atrophic, fibrous distal nerve roots in long-standing
myelodysplastic cases can be holding the conus tautly against
the dura and can thus prevent complete release of the tethering.
If these roots can be shown by the monitors to have no
contribution to sphincteric functions, they should be cut.
4. Occasionally, the junction between functional conus and fat
is not well demarcated in cases of large transitional lipomas or
the type of terminal lipoma not having an intervening filum
terminale. Direct stimulation proceeding from the obviously
normal portion of the conus in a caudal direction will identify
the lowest extent of pudendal motor neurons, beyond which
sphincter contractions can no longer be elicited by the nerve
stimulator (Figure 11).
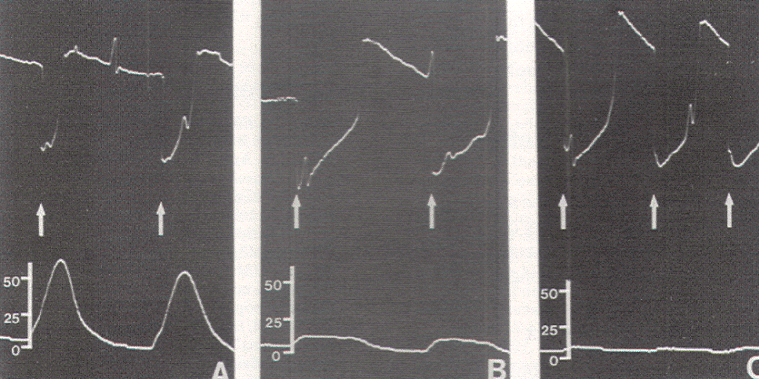
Figure 11.
Progressively caudal stimulation of the extremely stretched-out
conus of a 48-year-old patient with adult tethered cord
syndrome. Stimulation on the obviously normal portion of the
conus generated tall spike waves (A), stimulation at the
junctional zone between the conus and the filum produced smaller
waves with a wide base (B), and stimulation just beyond the
caudal extent of the conus elicited a minimal pressure response
(C). Arrows indicate stimulus artifacts. Scale in torr.
 Pelvic Floor EMG
Pelvic Floor EMG
Needle
recording electrodes can be percutaneously inserted into the
"extrinsic" portion of the external urethral sphincter to
monitor activity of this sphincter. This technique is
routinely used by neurourologists to correlate simultaneous
measurements of bladder pressure, urethral pressure, and
external urethral sphincter activities. Pelvic floor EMG can
thus be used for intraoperative sacral root identification
in the same manner as external anal sphincter EMG.
 Modality for Sacral Reflex Monitoring
Modality for Sacral Reflex Monitoring
Two
reflexes with centers in the sacral cord can be utilized to
assess the integrity of both the sensory and motor roots as
well as their interconnecting intramedullary components.
 Bulbocavernosus Reflex
Bulbocavernosus Reflex
The
reflex response of the bulbocavernosus muscle to stimulation
of penile nerves can be studied using square wave electrical
stimuli applied through ring electrodes on the penis, and
recorded either by needle electrodes in the muscle or by
surface electrodes fixed to the midline of the perineum,
between the base of the penis and the anus. The averaged
response from 50 to 100 stimuli is usually biphasic with an
initial negative peak. The latency for most healthy adults
is 24 to 42 msec but varies with age and maturation in young
children. The waveform is also distorted significantly in
most cases of myelodysplasia and tends to become "unstable"
with very minor manipulations of the conus. The use of this
monitoring modality is therefore limited and is feasible
only in patients with virtually normal sphincter function
preoperatively.
 Urethral to Anal Sphincter Reflex Response
Urethral to Anal Sphincter Reflex Response
The
urethral to anal sphincter reflex can be measured using
stimulating electrodes similar to those used in eliciting
urethral cortical evoked responses and recording electrodes
used in recording external anal sphincter EMG. The latency
is considerably longer (50 to 70 msec) than the
bulbocavernosus reflex, although their morphologies are
similar. The long latency in the urethral-anal sphincter
reflex is due partly to the slower conducting velocity of
autonomic afferent fibers and partly to a more complex
central polysynaptic reflex organization.
|